Shelterin
Shelterin (also called telosome) is a protein complex known to protect telomeres in many eukaryotes from DNA repair mechanisms, as well as regulate telomerase activity. In mammals and other eukaryotes, telomeric DNA consists of double- and single-stranded TTAGGG repeats and a single-stranded, G-rich overhang. Subunits of shelterin bind to these regions and induce the formation of a t-loop, a cap structure that deters DNA-damage-sensing machinery from mistakenly repairing telomeres. The absence of shelterin causes telomere uncapping and thereby activates damage-signaling pathways that may lead to non-homologous end joining (NHEJ), homology directed repair (HDR),[1] senescence, or apoptosis.[2]
Subunits
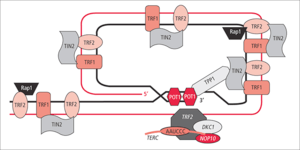
Shelterin has six subunits: TRF1, TRF2, POT1, RAP1, TIN2, and TPP1.[3] They can operate in smaller subsets to regulate the length of or protect telomeres.
- TRF1 (Telomere Repeat Factor 1): TRF1 is a homodimeric protein[4] that binds to the double-stranded TTAGGG region of the telomere. TRF1 along with TRF2 normally prevents telomerase from adding more telomere units to telomeres.[5] But when telomere lengthening is required, TRF1 recruits helicases to facilitate the process.[6] TRF1 may recruit PINX1 to inhibit telomere elongation by telomerase.[2]
- TRF2 (Telomere Repeat Factor 2): TRF2 is a homodimeric protein[4] that binds to the double-stranded TTAGGG region of the telomere and prevents the recognition of double-strand DNA breaks.[7]
- RAP1 (Repressor / Activator Protein 1): RAP1 is a stabilizing protein associated with TRF2.[8]
- POT1 (Protection of Telomere 1): POT1 contains OB-folds (oligonucleotide/oligosaccharide binding) that bind POT1 to single-stranded DNA,[9] which increase its affinity for single-stranded TTAGGG region of telomeric DNA. POT1 prevents the degradation of this single stranded DNA by nucleases and shelters the G-overhang.[3] Humans only have a single POT1, whereas mice have POT1a and POT1b.[10]
- TPP1 (Tripeptidyl-peptidase 1): TPP1 is a protein associated with POT1. The loss of TPP1 leads to impaired POT1 function.[2] When telomeres are to be lengthened, TPP1 is a central factor in recruiting telomerase to telomeres.[11]
- TIN2 (TRF1- and TRF2-Interacting Nuclear Protein 2): TIN2 is a stabilizing protein that binds to the TRF1, TRF2, and the TPP1-POT1 complex.[12] thereby bridging units attached to double-stranded DNA and units attached to single-stranded DNA.[2]
Repression of DNA repair mechanisms
There are two main DNA-damage-signaling pathways that shelterin represses: the ATR kinase pathway, blocked by POT1, and the ATM kinase pathway, blocked by TRF2.[4] In the ATR kinase pathway, ATR and ATRIP sense the presence of single-stranded DNA and induce a phosphorylation cascade that leads to cell cycle arrest. To prevent this signal, POT1 "shelters" the single-stranded region of telomeric DNA. The ATM kinase pathway, which starts from ATM and other proteins sensing double strand breaks, similarly ends with cell cycle arrest. TRF2 may also hide the ends of telomeres, just as POT1 hides the single-stranded regions. Another theory proposes the blocking of the signal downstream. This will lead to a dynamic instability of the cells over time.
The structure of the t-loop may prevent NHEJ.[4] For NHEJ to occur, the Ku heterodimer must be able to bind to the ends of the chromosome. Another theory offers the mechanism proposed earlier: TRF2 hides the ends of telomeres.[2]
Species differences
At least four factors contribute to telomere maintenance in most eurkaryotes: telomerase, shelterin, TERRA and the CST Complex.[13] Fission yeast (Schizosaccharomyces pombe) has a shelterin complex for protection and maintenance of telomeres, but in budding yeast (Saccharomyces cerevisiae) this function is performed by the CST Complex.[14] For fission yeast, Rap1 and Pot1 are conserved, but Tpz1 is an ortholog of TPP1 and Taz1 is an ortholog of TRF1 and TRF2.[15]
Plants contain a variety of telomere-protecting proteins which can resemble either shelterin or the CST Complex.[16]
The fruit fly Drosophila melanogaster lacks both shelterin and telomerase, but instead uses retrotransposons to maintain telomeres.[17]
Non-telomeric functions of shelterin proteins
TIN2 can localize to mitochondria where it promotes glycolysis.[18]
RAP1 regulates transcription and affects NF-κB signaling.[6]
See also
References
- ↑ Rodriguez, Raphaël, Sebastian Müller, Justin A. Yeoman, Chantal Trentesaux, Jean-Françios Riou, and Shankar Balasubramanian. "A Novel Small Molecule That Alters Shelterin Integrity and Triggers a DNA-Damage Response at Telomeres." Journal of the American Chemical Society 130 (2008): 15758-59. doi: 10.1021/ja805615w.
- 1 2 3 4 5 Palm, Wilhelm, and Titia de Lange. "How Shelterin Protects Mammalian Telomeres." Annual Reviews 42 (2008): 301-34. doi: 10.1146/annurev.genet.41.110306.130350.
- 1 2 Xin, Huawei, Dan Liu, and Zhou Songyang. "The telomere/shelterin complex and its functions." Genome Biology 9 (2008): 232.
- 1 2 3 4 de Lange, Titia. "How Shelterin Solves the Telomere End-Protection Problem." Cold Spring Harbor Symposia on Quantitative Biology 75 (2010): 167-77. doi: 10.1101/sqb.2010.75.017.
- ↑ Diotti R1, Loayza D (2011). "Shelterin complex and associated factors at human telomeres". NUCLEUS. 2 (2): 119–135. doi:10.4161/nucl.2.2.15135. PMC 3127094
 . PMID 21738835.
. PMID 21738835. - 1 2 Sfeir A (2012). "Telomeres at a glance". Journal of Cell Science. 125 (Pt 18): 4173–4178. doi:10.1242/jcs.106831. PMID 23135002.
- ↑ Choi, Kyung H., Amy S. Farrell, Amanda S. Lakamp, and Michel M. Ouellette. "Characterization of the DNA binding specificity of Shelterin complexes." Nucleic Acids Research 39 (2011): 9206-23. doi 10.1093/nar/gkr665.
- ↑ Nandakumar J1, Cech TR (2013). "Finding the end: recruitment of telomerase to telomeres". Nature Reviews Molecular Cell Biology. 14 (2): 69–82. doi:10.1038/nrm3505. PMC 3805138
 . PMID 23299958.
. PMID 23299958. - ↑ Flynn RL, Zou L (2010). "Oligonucleotide/oligosaccharide-binding fold proteins: a growing family of genome guardians". Critical Reviews in Biochemistry and Molecular Biology. 45 (4): 266–275. doi:10.3109/10409238.2010.488216. PMC 2906097
 . PMID 20515430.
. PMID 20515430. - ↑ Martínez P1, Blasco MA (2010). "Role of shelterin in cancer and aging". Aging Cell. 9 (5): 653–666. doi:10.1111/j.1474-9726.2010.00596.x. PMID 20569239.
- ↑ Abreu E1, Aritonovska E, Reichenbach P, Cristofari G, Culp B, Terns RM, Lingner J, Terns MP (2010). "TIN2-tethered TPP1 recruits human telomerase to telomeres in vivo". Molecular and Cellular Biology. 30 (12): 2971–2982. doi:10.1128/MCB.00240-10. PMC 2876666
 . PMID 20404094.
. PMID 20404094. - ↑ Takai, Kaori K., Sarah Hooper, Stephanie Blackwood, Rita Gandhi, and Titia de Lange. "In Vivo Stoichiometry of Shelterin Components." Journal of Biological Chemistry 285 (2010): 1457-67. doi: 10.1074/jbc.M109.038026.
- ↑ Giraud-Panis MJ, Teixeira MT, Géli V, Gilson E (2010). "CST meets shelterin to keep telomeres in check". Molecular Cell. 39 (5): 665–676. doi:10.1016/j.molcel.2010.08.024. PMID 20832719.
- ↑ Price CM, Boltz KA, Chaiken MF, Stewart JA, Beilstein MA, Shippen DE (2010). "Evolution of CST function in telomere maintenance". Cell Cycle (journal). 9 (16): 3157–3165. doi:10.4161/cc.9.16.12547. PMC 3041159
 . PMID 20697207.
. PMID 20697207. - ↑ Miyagawa K, Low RS, Santosa V, Tsuji H, Moser BA, Fujisawa S, Harland JL, Raguimova ON, Go A, Ueno M, Matsuyama A, Yoshida M, Nakamura TM, Tanaka K (2014). "SUMOylation regulates telomere length by targeting the shelterin subunit Tpz1(Tpp1) to modulate shelterin-Stn1 interaction in fission yeast" (PDF). PNAS. 111 (16): 5950–5955. doi:10.1073/pnas.1401359111. PMC 4000806
 . PMID 24711392.
. PMID 24711392. - ↑ Procházková Schrumpfová P, Schořová Š, Fajkus J (2016). "Telomere- and Telomerase-Associated Proteins and Their Functions in the Plant Cell". FRONTIERS IN PLANT SCIENCE. 7: 851. doi:10.3389/fpls.2016.00851. PMC 4924339
 . PMID 27446102.
. PMID 27446102. - ↑ Pardue ML, DeBaryshe PG (2011). "Retrotransposons that maintain chromosome ends" (PDF). PNAS. 108 (51): 20317–20324. doi:10.1073/pnas.1100278108. PMC 3251079
 . PMID 21821789.
. PMID 21821789. - ↑ Chen LY, Zhang Y, Zhang Q, Li H, Luo Z, Fang H, Kim SH, Qin L, Yotnda P, Xu J, Tu BP, Bai Y, Songyang Z (2012). "Mitochondrial localization of telomeric protein TIN2 links telomere regulation to metabolic control". Molecular Cell. 47 (6): 839–850. doi:10.1016/j.molcel.2012.07.002. PMC 3462252
 . PMID 22885005.
. PMID 22885005.