Disodium hydrogen phosphite
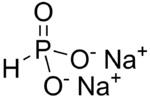 | |
 | |
| Names | |
|---|---|
| IUPAC name
sodium phosphonate pentahydrate | |
| Other names
Sodium phosphite dibasic pentahydrate, sodium phosphite | |
| Identifiers | |
| 13708-85-5 13517-23-2 (pentahydrate) | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChemSpider | 21106436 |
| ECHA InfoCard | 100.033.848 |
| |
| |
| Properties | |
| HNa2O3P | |
| Molar mass | 125.96 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Disodium hydrogen phosphite is the chemical compound Na2HPO3 which is commonly encountered as the pentahydrate. It is a salt of phosphorous acid, HP(O)(OH)2 and contains the anion HPO32−. Its common name suggests that it contains an acidic hydrogen atom, as in sodium hydrogen carbonate. However, this is misleading as the hydrogen atom is bonded to phosphorus rather than oxygen. It is toxic along with all phosphites. Phosphites contain phosphorus in its +3 oxidation state and are therefore reducing agents.
This article is issued from Wikipedia - version of the 7/7/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.