Spidroin
Spidroin is the main protein in a spider's dragline silk. It is part of a unique family of large structural proteins that make up the bulk of spider silk fibers.
There are two types of spidroin: spidroin 1 and spidroin 2. They differ in their percentages of specific amino acids.[1]
Spidroin is part of a large group ofRH57H90307F proteins called scleroproteins. This group includes other structural proteins such as collagen and keratin. The specific characteristics of spidroins are becoming more commonly researched.
A fiber of spidroin is as thick and resistant as one of steel but is more flexible. It can be stretched to approximately 135% of its original length without breaking. Its properties make it an excellent candidate for use in various scientific fields.[2]
Structure
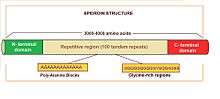
Major ampullate spidroins are large proteins with an extension of 250-350 kDa, with an average of 3500 amino acids. They represent a polymeric organization, mostly based on highly homogenized tandem repeats. There are 100 tandem copies of 30 to 40 amino acids which repeat sequence and they represent more than 90% of the protein sequence.[3] Alanine and glycine residues are the most abundant. Alanine appears in blocks of six to fourteen units that form β-sheets. These alanine blocks can stack to create crystalline structures in the fiber, linking different protein molecules together. Glycine is present in different motifs, such as GGX and GPGXX (where X = A, L, Q, or Y), that also have specific secondary structures (3 10 helix and β-spiral, respectively). Glycine-rich regions are more amorphous and contribute to extensibility and flexibility. Some of the differences observed between spidroin 1 and spidroin 2 (the most important major ampullate spidroins) are the proline content, which is very low in the first one but significant in the second one, and the motifs. Motif (GGX)n is characteristic in spidroin 1, while GPG and QQ are typical in spidroin 2.
On the other hand, spidroins have non-repetitive amino (N) and carboxyl (C) terminal domains of approximately 150 and 100 amino acids respectively. N- and C-terminal domains share little resemblance, except that they are both rich in serine and both are largely amphipathic α-helical secondary structures. These domains are conserved not only between spidroin 1 and 2, but also among many silk types and spider species. Experimental data show the N- and C-terminal domains contribute to fiber assembly.[4] The C-terminal domain is involved in the organized transition from a soluble spidroin solution to an insoluble fiber during spinning.[5] In the N-terminal domain, there are signal peptides which regulate spidroin secretion from silk gland cells.[6][7]
Biological function
An individual spider spins a multitude of silk types, with each type emerging from its own distinctive set of abdominal silk glands. This complex silk machinery enables spiders to use task-specific silks (e.g., for web assembly, egg-case construction, prey wrapping, etc.).[8] The different types of silk (major ampulate silk, minor ampulate silk, flagelliform silk, aciniform silk, tubiliform silk, pyriform silk, and aggregate silk)[9] are composed of different types of proteins.
Dragline silk is mainly formed by spidroin proteins. It is a type of major ampulate silk and is produced in the major ampulate gland. Dragline silk is used not only to construct the outer frame and radii of the orb-shaped web but also as a hanging lifeline that allows the spider to evade and/or escape from predators.[10] The major ampulate gland that produces this silk is formed by three main sections: a central bag (B zone) flanked by a tail (A zone) and a duct heading towards the exit. The tail secretes most of the “spinning dope”, a solution which contains the protein molecules that will constitute the silk fiber. The sac is the main storage repository.
The epithelium of the A zone is composed of tall columns of secretory cells of a single type, packed with secretory granules. The major component of these cells which secrete the fibroin solution is a 275kDa protein containing the polypeptides spidroin I and spidroin II. The output of these cells is an aquous and highly viscous solution of about 50% protein (mostly spidroin). The product secreted makes up the dragline silk, the main structure.
This highly viscous protein emulsion flows into the B zone, where it is covered by glycoproteins. After exiting this bag, the liquid is funneled into the narrow duct. As the gelatinous protein solution moves into the duct, the integral spidroins and glycoproteins are gradually distorted into long, thin, aligned figures with the direction of the flow. Then, they are stretched and lined up in a way that will eventually allow them to create strong intermolecular links. After different processes the silk is extended in the spinning channel to form an extremely tough thread.
Industrial and biomedical applications
In the last decade, much research has been done about spidroin protein and spider silk in order to take advantage of some of its properties, such as its elasticity and strength. Spider silk is used in different industries, and its range of applications in biomedicine is increasing every day. For example, the military and defense industries use bulletproof vests made from these fibers.
Recombinant spidroin has been successfully obtained in both eukaryotic and prokaryotic cells although there were some difficulties in the procedure due to the length of the gene sequence. Thanks to expression and the cloning work, it is possible to obtain large-scale production of spidroin which provides new opportunities for the manufacture of new biomaterials.[11] There have been attempts to generate transgenic tobacco and potato plants that express remarkable amounts of recombinant Nephila clavipes dragline proteins.[12]
Furthermore, fibers developed from spidroin are tolerated in vitro, in cell culture, and in vivo, in animals like pigs, as no signs of either inflammatory response nor body reaction were shown to these fibers. These results suggest that they could be used in medicine without risk of biocompatibility issues and thus potentially lead to many new opportunities in tissue engineering and regenerative medicine.
See also
References
- ↑ Moisenovich, M; Pustovalova, O; Shackelford, J; Vasiljeva, T (2012). "Tissue regeneration in vivo within recombinant spidroin 1 scaffolds". Biomaterials. 33: 3887–3898. doi:10.1016/j.biomaterials.2012.02.013.
- ↑ Askarieh, G; Hedhammar, M; Nordling, K; Saenz, A; Casals, C; Rising, A; Johansson, J; Knight, S. "Self-assembly of spider silk proteins is controlled by a pH-sensitive relay". Nature. 465: 236–238. doi:10.1038/nature08962.
- ↑ Xu, M; Lewis, RV (1990). "Structure of a protein superfiber: spider dragline silk". Proc Natl Acad Sci USA. 87: 7120–7124. doi:10.1073/pnas.87.18.7120.
- ↑ Huemmerich, D; Helsen, CW; Quedzuweit, S; Oschmann JRudolph, R; Scheibel, T (2004). "Primary structure elements of spider dragline silks and their contribution to protein solubility". Biochemistry. 43: 13604–13612. doi:10.1021/bi048983q.
- ↑ Sponner, A; Vater, W; Rommerskirch, W; Vollrath, F; Unger, E; Grosse, F; Weisshart, K (2005). "The conserved C-termini contribute to the properties of spider silk fibroins". Biochem Biophys Res Commun. 338: 897–902. doi:10.1016/j.bbrc.2005.10.048. PMID 16253207.
- ↑ Stark, M; Grip, S; Rising, A; Hedhammar, M; Engstrom, W; Hjalm, G; Johansson, J (2007). "Macroscopic fibers self-assembled from recombinant miniature spider silk proteins". Biomacromolecules. 8: 1695–1701. doi:10.1021/bm070049y. PMID 17402782.
- ↑ Garb, J; Ayoub, N; Hayashi, C. "Untangling spider silk evolution with spidroin terminal domains". BMC Evolutionary Biology. 2010 (10): 243.
- ↑ http://www.biomedcentral.com/1471-2148/10/243
- ↑ Howorka S, editor. Progress in molecular biology and translational science (103). London: Elsevier Science; 2011.
- ↑ Gaines, WA; Marcotte, WR (2008). "Identification and characterization of multiple Spidroin 1 genes encoding major ampullate silk proteins in Nephila clavipes". Insect Mol. Biol. 17: 465–74. doi:10.1111/j.1365-2583.2008.00828.x. PMC 2831225
 . PMID 18828837.
. PMID 18828837. - ↑ Rising A, Widhe M, Johansson J, Hedhammar M. Spider silk proteins: recent advances in recombinant production,structure–function relationships and biomedical applications. Cell. Mol. Life Sci. (2011) 68:169–184
- ↑ Scheller, J.; Guhrs, K.H.; Grosse, F.; Conrad, U. (2001). "Production of spider silk proteins in tobacco and potato". Nature Biotechnology. 19: 573–577.
External links
- http://www.uniprot.org/uniprot/P19837
- http://www.uniprot.org/uniprot/P46804
- http://www.chm.bris.ac.uk/motm/spider/page3.htm
- http://web.expasy.org/spotlight/snapshots/047/
- http://www.nature.com/nature/journal/v465/n7295/fig_tab/nature08962_F2.html
- http://www.rcsb.org/pdb/explore/explore.do?structureId=3LR2