Superoxide dismutase
| Superoxide dismutase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
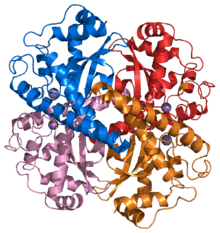 Structure of a human Mn superoxide dismutase 2 tetramer.[1] | |||||||||
| Identifiers | |||||||||
| EC number | 1.15.1.1 | ||||||||
| CAS number | 9054-89-1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
Superoxide dismutase (SOD, EC 1.15.1.1) is an enzyme that alternately catalyzes the dismutation (or partitioning) of the superoxide (O2−) radical into either ordinary molecular oxygen (O2) or hydrogen peroxide (H2O2). Superoxide is produced as a by-product of oxygen metabolism and, if not regulated, causes many types of cell damage.[2] Hydrogen peroxide is also damaging, but less so, and is degraded by other enzymes such as catalase. Thus, SOD is an important antioxidant defense in nearly all living cells exposed to oxygen. One exception is Lactobacillus plantarum and related lactobacilli, which use a different mechanism to prevent damage from reactive (O2−).
Chemical reaction
SOD enzymes deal with the superoxide radical by alternately adding or removing an electron from the superoxide molecules it encounters, thus changing the O2− into one of two less damaging species: either molecular oxygen (O2) or hydrogen peroxide (H2O2). This SOD-catalyzed dismutation of superoxide may be written, for Cu,Zn SOD, with the following half-reactions :
- Cu2+-SOD + O2− → Cu+-SOD + O2
- Cu+-SOD + O2− + 2H+ → Cu2+-SOD + H2O2
The general form, applicable to all the different metal-coordinated forms of SOD, can be written as follows:
- M(n+1)+-SOD + O2− → Mn+-SOD + O2
- Mn+-SOD + O2− + 2H+ → M(n+1)+-SOD + H2O2.
where M = Cu (n=1) ; Mn (n=2) ; Fe (n=2) ; Ni (n=2).
In a series of such reactions, the oxidation state and the charge of the metal cation oscillates between n and n+1: +1 and +2 for Cu, or +2 and +3 for the other metals .
Types
General
Irwin Fridovich and Joe McCord at Duke University discovered the enzymatic activity of superoxide dismutase in 1968.[3] SODs were previously known as a group of metalloproteins with unknown function; for example, CuZnSOD was known as erythrocuprein (or hemocuprein, or cytocuprein) or as the veterinary anti-inflammatory drug "Orgotein".[4] Likewise, Brewer (1967) identified a protein that later became known as superoxide dismutase as an indophenol oxidase by protein analysis of starch gels using the phenazine-tetrazolium technique.[5]
There are three major families of superoxide dismutase, depending on the protein fold and the metal cofactor: the Cu/Zn type (which binds both copper and zinc), Fe and Mn types (which bind either iron or manganese), and the Ni type (which binds nickel).
 Ribbon diagram of bovine Cu-Zn SOD subunit[6] |
 Active site of Human Manganese SOD, manganese shown in purple[7] |
 Mn-SOD vs Fe-SOD dimers |
- Copper and zinc – most commonly used by eukaryotes, including humans. The cytosols of virtually all eukaryotic cells contain an SOD enzyme with copper and zinc (Cu-Zn-SOD). For example, Cu-Zn-SOD available commercially is normally purified from bovine red blood cells. The bovine Cu-Zn enzyme is a homodimer of molecular weight 32,500. It was the first SOD whose atomic-detail crystal structure was solved, in 1975.[8] It is an 8-stranded "Greek key" beta-barrel, with the active site held between the barrel and two surface loops. The two subunits are tightly joined back-to-back, mostly by hydrophobic and some electrostatic interactions. The ligands of the copper and zinc are six histidine and one aspartate side-chains; one histidine is bound between the two metals.[9]
- Iron or manganese – used by prokaryotes and protists, and in mitochondria and chloroplasts
- Iron – Many bacteria contain a form of the enzyme with iron (Fe-SOD); some bacteria contain Fe-SOD, others Mn-SOD, and some (such as E. coli) contain both. Fe-SOD can also be found in the chloroplasts of plants. The 3D structures of the homologous Mn and Fe superoxide dismutases have the same arrangement of alpha-helices, and their active sites contain the same type and arrangement of amino acid side-chains. They are usually dimers, but occasionally tetramers.
- Manganese – Nearly all mitochondria, and many bacteria, contain a form with manganese (Mn-SOD): For example, the Mn-SOD found in human mitochondria. The ligands of the manganese ions are 3 histidine side-chains, an aspartate side-chain and a water molecule or hydroxy ligand, depending on the Mn oxidation state (respectively II and III).[10]
- Nickel – prokaryotic. This has a hexameric (6-copy) structure built from right-handed 4-helix bundles, each containing N-terminal hooks that chelate a Ni ion. The Ni-hook contains the motif His-Cys-X-X-Pro-Cys-Gly-X-Tyr; it provides most of the interactions critical for metal binding and catalysis and is, therefore, a likely diagnostic of NiSODs.[11][12]
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In higher plants, SOD isozymes have been localized in different cell compartments. Mn-SOD is present in mitochondria and peroxisomes. Fe-SOD has been found mainly in chloroplasts but has also been detected in peroxisomes, and CuZn-SOD has been localized in cytosol, chloroplasts, peroxisomes, and apoplast.[14][15]
Human
Three forms of superoxide dismutase are present in humans, in all other mammals, and most chordates. SOD1 is located in the cytoplasm, SOD2 in the mitochondria, and SOD3 is extracellular. The first is a dimer (consists of two units), whereas the others are tetramers (four subunits). SOD1 and SOD3 contain copper and zinc, whereas SOD2, the mitochondrial enzyme, has manganese in its reactive centre. The genes are located on chromosomes 21, 6, and 4, respectively (21q22.1, 6q25.3 and 4p15.3-p15.1).
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Plants
In higher plants, superoxide dismutase enzymes (SODs) act as antioxidants and protect cellular components from being oxidized by reactive oxygen species (ROS).[18] ROS can form as a result of drought, injury, herbicides and pesticides, ozone, plant metabolic activity, nutrient deficiencies, photoinhibition, temperature above and below ground, toxic metals, and UV or gamma rays.[19][20] To be specific, molecular O2 is reduced to O2− (an ROS called superoxide) when it absorbs an excited electron released from compounds of the electron transport chain. Superoxide is known to denature enzymes, oxidize lipids, and fragment DNA.[19] SODs catalyze the production of O2 and H2O2 from superoxide (O2−), which results in less harmful reactants.
When acclimating to increased levels of oxidative stress, SOD concentrations typically increase with the degree of stress conditions. The compartmentalization of different forms of SOD throughout the plant makes them counteract stress very effectively. There are three well-known and -studied classes of SOD metallic coenzymes that exist in plants. First, Fe SODs consist of two species, one homodimer (containing 1-2 g Fe) and one tetramer (containing 2-4 g Fe). They are thought to be the most ancient SOD metalloenzymes and are found within both prokaryotes and eukaryotes. Fe SODs are most abundantly localized inside plant chloroplasts, where they are indigenous. Second, Mn SODs consist of a homodimer and homotetramer species each containing a single Mn(III) atom per subunit. They are found predominantly in mitochondrion and peroxisomes. Third, Cu-Zn SODs have electrical properties very different from those of the other two classes. These are concentrated in the chloroplast, cytosol, and in some cases the extracellular space. Note that Cu-Zn SODs provide less protection than Fe SODs when localized in the chloroplast.[18][19][20]
Bacteria
Human white blood cells use enzymes such as NADPH oxidase to generate superoxide and other reactive oxygen species to kill bacteria. During infection, some bacteria (e.g., Burkholderia pseudomallei) therefore produce superoxide dismutase to protect themselves from being killed.[21]
Biochemistry
SOD out-competes damaging reactions of superoxide, thus protecting the cell from superoxide toxicity. The reaction of superoxide with non-radicals is spin-forbidden. In biological systems, this means that its main reactions are with itself (dismutation) or with another biological radical such as nitric oxide (NO) or with a transition-series metal. The superoxide anion radical (O2−) spontaneously dismutes to O2 and hydrogen peroxide (H2O2) quite rapidly (~105 M−1s−1 at pH 7). SOD is necessary because superoxide reacts with sensitive and critical cellular targets. For example, it reacts with the NO radical, and makes toxic peroxynitrite.
Because the uncatalysed dismutation reaction for superoxide requires two superoxide molecules to react with each other, the dismutation rate is second-order with respect to initial superoxide concentration. Thus, the half-life of superoxide, although very short at high concentrations (e.g., 0.05 seconds at 0.1mM) is actually quite long at low concentrations (e.g., 14 hours at 0.1 nM). In contrast, the reaction of superoxide with SOD is first order with respect to superoxide concentration. Moreover, superoxide dismutase has the largest kcat/KM (an approximation of catalytic efficiency) of any known enzyme (~7 x 109 M−1s−1),[22] this reaction being limited only by the frequency of collision between itself and superoxide. That is, the reaction rate is "diffusion-limited".
The high efficiency of superoxide dismutase seems necessary: even at the subnanomolar concentrations achieved by the high concentrations of SOD within cells, superoxide inactivates the citric acid cycle enzyme aconitase, can poison energy metabolism, and releases potentially toxic iron. Aconitase is one of several iron-sulfur-containing (de)hydratases in metabolic pathways shown to be inactivated by superoxide.[23]
Physiology
Superoxide is one of the main reactive oxygen species in the cell. As a consequence, SOD serves a key antioxidant role. The physiological importance of SODs is illustrated by the severe pathologies evident in mice genetically engineered to lack these enzymes. Mice lacking SOD2 die several days after birth, amid massive oxidative stress.[24] Mice lacking SOD1 develop a wide range of pathologies, including hepatocellular carcinoma,[25] an acceleration of age-related muscle mass loss,[26] an earlier incidence of cataracts and a reduced lifespan. Mice lacking SOD3 do not show any obvious defects and exhibit a normal lifespan, though they are more sensitive to hyperoxic injury.[27] Knockout mice of any SOD enzyme are more sensitive to the lethal effects of superoxide-generating compounds, such as paraquat and diquat (herbicides).
Drosophila lacking SOD1 have a dramatically shortened lifespan, whereas flies lacking SOD2 die before birth. SOD knockdowns in C. elegans do not cause major physiological disruptions. Knockout or null mutations in SOD1 are highly detrimental to aerobic growth in the yeast Saccharomyces cerevisiae and result in a dramatic reduction in post-diauxic lifespan. SOD2 knockout or null mutations cause growth inhibition on respiratory carbon sources in addition to decreased post-diauxic lifespan.
Several prokaryotic SOD null mutants have been generated, including E. coli. The loss of periplasmic CuZnSOD causes loss of virulence and might be an attractive target for new antibiotics.
Role in disease
Mutations in the first SOD enzyme (SOD1) can cause familial amyotrophic lateral sclerosis (ALS, a form of motor neuron disease).[28][29][30][31] The most common mutation in the U.S. is A4V, while the most intensely studied is G93A. The other two isoforms of SOD have not been linked to any human diseases, however, in mice inactivation of SOD2 causes perinatal lethality[24] and inactivation of SOD1 causes hepatocellular carcinoma.[25] Mutations in SOD1 can cause familial ALS (several pieces of evidence also show that wild-type SOD1, under conditions of cellular stress, is implicated in a significant fraction of sporadic ALS cases, which represent 90% of ALS patients.),[32] by a mechanism that is presently not understood, but not due to loss of enzymatic activity or a decrease in the conformational stability of the SOD1 protein. Overexpression of SOD1 has been linked to the neural disorders seen in Down syndrome.[33] In patients with thalassemia, SOD will increase as a form of compensation mechanism. However, in the chronic stage, SOD does not seem to be insufficient and tends to decrease due to the destruction of proteins from the massive reaction of oxidant-antioxidant.[34]
In mice, the extracellular superoxide dismutase (SOD3, ecSOD) contributes to the development of hypertension.[35][36] Diminished SOD3 activity has been linked to lung diseases such as Acute Respiratory Distress Syndrome (ARDS) or Chronic obstructive pulmonary disease (COPD).[37][38][39]
Superoxide dismutase is also not expressed in neural crest cells in the developing fetus. Hence, high levels of free radicals can cause damage to them and induce dysraphic anomalies (neural tube defects).
Pharmacological activity
SOD has powerful antinflammatory activity. For example, SOD is a highly effective experimental treatment of chronic inflammation in colitis. Treatment with SOD decreases reactive oxygen species generation and oxidative stress and, thus, inhibits endothelial activation and indicate that modulation of factors that govern adhesion molecule expression and leukocyte-endothelial interactions. Therefore, such antioxidants may be important new therapies for the treatment of inflammatory bowel disease.[40]
Likewise, SOD has multiple pharmacological activities. E.g., it ameliorates cis-platinum-induced nephrotoxicity in rodents.[41] As "Orgotein" or "ontosein", a pharmacologically-active purified bovine liver SOD, it is also effective in the treatment of urinary tract inflammatory disease in man.[42] For a time, bovine liver SOD even had regulatory approval in several European countries for such use. This was truncated by concerns about prion disease.
An SOD-mimetic agent, TEMPOL, is currently in clinical trials for radioprotection and to prevent radiation-induced dermatitis.[43] TEMPOL and similar SOD-mimetic nitroxides exhibit a multiplicity of actions in diseases involving oxidative stress.[44]
Cosmetic uses
SOD may reduce free radical damage to skin—for example, to reduce fibrosis following radiation for breast cancer. Studies of this kind must be regarded as tentative, however, as there were not adequate controls in the study including a lack of randomization, double-blinding, or placebo.[45] Superoxide dismutase is known to reverse fibrosis, perhaps through reversion of myofibroblasts back to fibroblasts.[46]
Commercial sources
SOD is commercially obtained from marine phytoplankton,bovine liver, horseradish, cantaloupe and by fermenting certain bacteria, though it is found in most living forms at diverse concentrations. For therapeutic purpose, SOD is usually injected locally. There is no evidence that ingestion of unprotected SOD or SOD-rich foods can have any physiological effects: as all ingested SOD is broken down into amino acids before being absorbed. However, ingestion of SOD bound to wheat proteins could improve its therapeutic activity, at least in theory.[47]
See also
- Catalase
- Peroxidase
- Jiaogulan
- NADPH oxidase, an enzyme which produces superoxide
References
- 1 2 PDB: 1VAR; Borgstahl GE, Parge HE, Hickey MJ, Johnson MJ, Boissinot M, Hallewell RA, Lepock JR, Cabelli DE, Tainer JA (April 1996). "Human mitochondrial manganese superoxide dismutase polymorphic variant Ile58Thr reduces activity by destabilizing the tetrameric interface". Biochemistry. 35 (14): 4287–97. doi:10.1021/bi951892w. PMID 8605177.
- ↑ Hayyan M., Hashim M.A., AlNashef I.M., Superoxide Ion: Generation and Chemical Implications, Chem. Rev., 2016, 116 (5), pp 3029–3085. DOI: 10.1021/acs.chemrev.5b00407
- ↑ McCord JM, Fridovich I (Nov 1969). "Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein)". The Journal of Biological Chemistry. 244 (22): 6049–55. PMID 5389100.
- ↑ McCord JM, Fridovich I (1988). "Superoxide dismutase: the first twenty years (1968-1988)". Free Radical Biology & Medicine. 5 (5-6): 363–9. doi:10.1016/0891-5849(88)90109-8. PMID 2855736.
- ↑ Brewer GJ (Sep 1967). "Achromatic regions of tetrazolium stained starch gels: inherited electrophoretic variation". American Journal of Human Genetics. 19 (5): 674–80. PMC 1706241
 . PMID 4292999.
. PMID 4292999. - ↑ PDB: 2SOD;Tainer JA, Getzoff ED, Beem KM, Richardson JS, Richardson DC (September 1982). "Determination and analysis of the 2 A-structure of copper, zinc superoxide dismutase". J. Mol. Biol. 160 (2): 181–217. doi:10.1016/0022-2836(82)90174-7. PMID 7175933.
- ↑ Quint P, Reutzel R, Mikulski R, McKenna R, Silverman DN (Feb 2006). "Crystal structure of nitrated human manganese superoxide dismutase: mechanism of inactivation". Free Radical Biology & Medicine. 40 (3): 453–8. doi:10.1016/j.freeradbiomed.2005.08.045. PMID 16443160.
- ↑ Richardson J, Thomas KA, Rubin BH, Richardson DC (Apr 1975). "Crystal structure of bovine Cu,Zn superoxide dismutase at 3 A resolution: chain tracing and metal ligands". Proceedings of the National Academy of Sciences of the United States of America. 72 (4): 1349–53. doi:10.1073/pnas.72.4.1349. PMC 432531
 . PMID 1055410..
. PMID 1055410.. - ↑ Tainer JA, Getzoff ED, Richardson JS, Richardson DC (1983). "Structure and mechanism of copper, zinc superoxide dismutase". Nature. 306 (5940): 284–7. doi:10.1038/306284a0. PMID 6316150.
- 1 2 3 PDB: 1N0J; Borgstahl GE, Parge HE, Hickey MJ, Beyer WF, Hallewell RA, Tainer JA (Oct 1992). "The structure of human mitochondrial manganese superoxide dismutase reveals a novel tetrameric interface of two 4-helix bundles". Cell. 71 (1): 107–18. doi:10.1016/0092-8674(92)90270-M. PMID 1394426.
- ↑ Barondeau DP, Kassmann CJ, Bruns CK, Tainer JA, Getzoff ED (Jun 2004). "Nickel superoxide dismutase structure and mechanism". Biochemistry. 43 (25): 8038–47. doi:10.1021/bi0496081. PMID 15209499.
- 1 2 PDB: 1Q0M; Wuerges J, Lee JW, Yim YI, Yim HS, Kang SO, Djinovic Carugo K (Jun 2004). "Crystal structure of nickel-containing superoxide dismutase reveals another type of active site". Proceedings of the National Academy of Sciences of the United States of America. 101 (23): 8569–74. doi:10.1073/pnas.0308514101. PMC 423235
 . PMID 15173586.
. PMID 15173586. - ↑ PDB: 1SDY; Djinović K, Gatti G, Coda A, Antolini L, Pelosi G, Desideri A, Falconi M, Marmocchi F, Rolilio G, Bolognesi M (December 1991). "Structure solution and molecular dynamics refinement of the yeast Cu,Zn enzyme superoxide dismutase". Acta Crystallogr. B. 47 (6): 918–27. doi:10.1107/S0108768191004949. PMID 1772629.
- ↑ Corpas FJ, Barroso JB, del Río LA (Apr 2001). "Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells". Trends in Plant Science. 6 (4): 145–50. doi:10.1016/S1360-1385(01)01898-2. PMID 11286918.
- ↑ Corpas FJ, Fernández-Ocaña A, Carreras A, Valderrama R, Luque F, Esteban FJ, Rodríguez-Serrano M, Chaki M, Pedrajas JR, Sandalio LM, del Río LA, Barroso JB (Jul 2006). "The expression of different superoxide dismutase forms is cell-type dependent in olive (Olea europaea L.) leaves". Plant & Cell Physiology. 47 (7): 984–94. doi:10.1093/pcp/pcj071. PMID 16766574.
- ↑ PDB: 3CQQ; Cao X, Antonyuk SV, Seetharaman SV, Whitson LJ, Taylor AB, Holloway SP, Strange RW, Doucette PA, Valentine JS, Tiwari A, Hayward LJ, Padua S, Cohlberg JA, Hasnain SS, Hart PJ (June 2008). "Structures of the G85R variant of SOD1 in familial amyotrophic lateral sclerosis". J. Biol. Chem. 283 (23): 16169–77. doi:10.1074/jbc.M801522200. PMC 2414278
 . PMID 18378676.
. PMID 18378676. - ↑ PDB: 2JLP; Antonyuk SV, Strange RW, Marklund SL, Hasnain SS (May 2009). "The structure of human extracellular copper-zinc superoxide dismutase at 1.7 A resolution: insights into heparin and collagen binding". J. Mol. Biol. 388 (2): 310–26. doi:10.1016/j.jmb.2009.03.026. PMID 19289127.
- 1 2 Alscher RG, Erturk N, Heath LS (May 2002). "Role of superoxide dismutases (SODs) in controlling oxidative stress in plants". Journal of Experimental Botany. 53 (372): 1331–41. doi:10.1093/jexbot/53.372.1331. PMID 11997379.
- 1 2 3 Smirnoff, Nicholas (1993). "Tansley Review No. 52 The role of active oxygen in the response of plants to water deficit and desiccation". Plant Phytology. 125.
- 1 2 Raychaudhuri SS, Deng XW (2008). "The Role of Superoxide Dismutase in Combating Oxidative Stress in Higher Plants". The Botanical Review. 66 (1): 89–98. doi:10.1007/BF02857783.
- ↑ Vanaporn M, Wand M, Michell SL, Sarkar-Tyson M, Ireland P, Goldman S, Kewcharoenwong C, Rinchai D, Lertmemongkolchai G, Titball RW (Aug 2011). "Superoxide dismutase C is required for intracellular survival and virulence of Burkholderia pseudomallei". Microbiology. 157 (Pt 8): 2392–400. doi:10.1099/mic.0.050823-0. PMID 21659326.
- ↑ Heinrich PC, Löffler G, Petrifies PE (2006). Biochemie und Pathobiochemie (Springer-Lehrbuch) (German Edition). Berlin: Springer. p. 123. ISBN 3-540-32680-4.
- ↑ Gardner PR, Raineri I, Epstein LB, White CW (Jun 1995). "Superoxide radical and iron modulate aconitase activity in mammalian cells". The Journal of Biological Chemistry. 270 (22): 13399–405. doi:10.1074/jbc.270.22.13399. PMID 7768942.
- 1 2 Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ (Dec 1995). "Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase". Nature Genetics. 11 (4): 376–81. doi:10.1038/ng1295-376. PMID 7493016.
- 1 2 Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H, Epstein CJ, Huang TT (Jan 2005). "CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life". Oncogene. 24 (3): 367–80. doi:10.1038/sj.onc.1208207. PMID 15531919.
- ↑ Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, Huang TT, Epstein CJ, Roberts LJ, Csete M, Faulkner JA, Van Remmen H (Jun 2006). "Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy". Free Radical Biology & Medicine. 40 (11): 1993–2004. doi:10.1016/j.freeradbiomed.2006.01.036. PMID 16716900.
- ↑ Sentman ML, Granström M, Jakobson H, Reaume A, Basu S, Marklund SL (Mar 2006). "Phenotypes of mice lacking extracellular superoxide dismutase and copper- and zinc-containing superoxide dismutase". The Journal of Biological Chemistry. 281 (11): 6904–9. doi:10.1074/jbc.M510764200. PMID 16377630.
- ↑ Milani P, Gagliardi S, Cova E, Cereda C (2011). "SOD1 Transcriptional and Posttranscriptional Regulation and Its Potential Implications in ALS". Neurology Research International. 2011: 458427. doi:10.1155/2011/458427. PMC 3096450
 . PMID 21603028.
. PMID 21603028. - ↑ Deng HX, Hentati A, Tainer JA, Iqbal Z, Cayabyab A, Hung WY, Getzoff ED, Hu P, Herzfeldt B, Roos RP (Aug 1993). "Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase". Science. 261 (5124): 1047–51. doi:10.1126/science.8351519. PMID 8351519.
- ↑ Conwit RA (Dec 2006). "Preventing familial ALS: a clinical trial may be feasible but is an efficacy trial warranted?". Journal of the Neurological Sciences. 251 (1-2): 1–2. doi:10.1016/j.jns.2006.07.009. PMID 17070848.
- ↑ Al-Chalabi A, Leigh PN (Aug 2000). "Recent advances in amyotrophic lateral sclerosis". Current Opinion in Neurology. 13 (4): 397–405. doi:10.1097/00019052-200008000-00006. PMID 10970056.
- ↑ Gagliardi S, Cova E, Davin A, Guareschi S, Abel K, Alvisi E, Laforenza U, Ghidoni R, Cashman JR, Ceroni M, Cereda C (Aug 2010). "SOD1 mRNA expression in sporadic amyotrophic lateral sclerosis". Neurobiology of Disease. 39 (2): 198–203. doi:10.1016/j.nbd.2010.04.008. PMID 20399857.
- ↑ Groner Y, Elroy-Stein O, Avraham KB, Schickler M, Knobler H, Minc-Golomb D, Bar-Peled O, Yarom R, Rotshenker S (1994). "Cell damage by excess CuZnSOD and Down's syndrome". Biomedicine & Pharmacotherapy = Biomédecine & Pharmacothérapie. 48 (5-6): 231–40. doi:10.1016/0753-3322(94)90138-4. PMID 7999984.
- ↑ Rujito L, Mulatsih S, Sofro AS (May 2015). "Status of Superoxide Dismutase in Transfusion Dependent Thalassaemia". North American Journal of Medical Sciences. 7 (5): 194–8. doi:10.4103/1947-2714.157480. PMID 26110130.
- ↑ Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, Harrison DG (Sep 2006). "Role of extracellular superoxide dismutase in hypertension". Hypertension. 48 (3): 473–81. doi:10.1161/01.HYP.0000235682.47673.ab. PMID 16864745.
- ↑ Lob HE, Marvar PJ, Guzik TJ, Sharma S, McCann LA, Weyand C, Gordon FJ, Harrison DG (Feb 2010). "Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system". Hypertension. 55 (2): 277–83, 6p following 283. doi:10.1161/HYPERTENSIONAHA.109.142646. PMC 2813894
 . PMID 20008675.
. PMID 20008675. - ↑ Young RP, Hopkins R, Black PN, Eddy C, Wu L, Gamble GD, Mills GD, Garrett JE, Eaton TE, Rees MI (May 2006). "Functional variants of antioxidant genes in smokers with COPD and in those with normal lung function". Thorax. 61 (5): 394–9. doi:10.1136/thx.2005.048512. PMC 2111196
 . PMID 16467073.
. PMID 16467073. - ↑ Ganguly K, Depner M, Fattman C, Bein K, Oury TD, Wesselkamper SC, Borchers MT, Schreiber M, Gao F, von Mutius E, Kabesch M, Leikauf GD, Schulz H (May 2009). "Superoxide dismutase 3, extracellular (SOD3) variants and lung function". Physiological Genomics. 37 (3): 260–7. doi:10.1152/physiolgenomics.90363.2008. PMC 2685504
 . PMID 19318538.
. PMID 19318538. - ↑ Gongora MC, Lob HE, Landmesser U, Guzik TJ, Martin WD, Ozumi K, Wall SM, Wilson DS, Murthy N, Gravanis M, Fukai T, Harrison DG (Oct 2008). "Loss of extracellular superoxide dismutase leads to acute lung damage in the presence of ambient air: a potential mechanism underlying adult respiratory distress syndrome". The American Journal of Pathology. 173 (4): 915–26. doi:10.2353/ajpath.2008.080119. PMC 2543061
 . PMID 18787098.
. PMID 18787098. - ↑ Seguí J, Gironella M, Sans M, Granell S, Gil F, Gimeno M, Coronel P, Piqué JM, Panés J (Sep 2004). "Superoxide dismutase ameliorates TNBS-induced colitis by reducing oxidative stress, adhesion molecule expression, and leukocyte recruitment into the inflamed intestine". Journal of Leukocyte Biology. 76 (3): 537–44. doi:10.1189/jlb.0304196. PMID 15197232.
- ↑ McGinness JE, Proctor PH, Demopoulos HB, Hokanson JA, Kirkpatrick DS (1978). "Amelioration of cis-platinum nephrotoxicity by orgotein (superoxide dismutase)". Physiological Chemistry and Physics. 10 (3): 267–77. PMID 733940.
- ↑ Marberger H, Huber W, Bartsch G, Schulte T, Swoboda P (1974). "Orgotein: a new antiinflammatory metalloprotein drug evaluation of clinical efficacy and safety in inflammatory conditions of the urinary tract". International Urology and Nephrology. 6 (2): 61–74. doi:10.1007/bf02081999. PMID 4615073.
- ↑ Clinical trial number NCT01324141 for "Topical MTS-01 for Dermatitis During Radiation and Chemotherapy for Anal Cancer" at ClinicalTrials.gov
- ↑ Wilcox CS (May 2010). "Effects of tempol and redox-cycling nitroxides in models of oxidative stress". Pharmacology & Therapeutics. 126 (2): 119–45. doi:10.1016/j.pharmthera.2010.01.003. PMC 2854323
 . PMID 20153367.
. PMID 20153367. - ↑ Campana F, Zervoudis S, Perdereau B, Gez E, Fourquet A, Badiu C, Tsakiris G, Koulaloglou S (2004). "Topical superoxide dismutase reduces post-irradiation breast cancer fibrosis". Journal of Cellular and Molecular Medicine. 8 (1): 109–16. doi:10.1111/j.1582-4934.2004.tb00265.x. PMID 15090266.
- ↑ Vozenin-Brotons MC, Sivan V, Gault N, Renard C, Geffrotin C, Delanian S, Lefaix JL, Martin M (Jan 2001). "Antifibrotic action of Cu/Zn SOD is mediated by TGF-beta1 repression and phenotypic reversion of myofibroblasts". Free Radical Biology & Medicine. 30 (1): 30–42. doi:10.1016/S0891-5849(00)00431-7. PMID 11134893.
- ↑ Romao S (Mar 2015). "Therapeutic value of oral supplementation with melon superoxide dismutase and wheat gliadin combination". Nutrition. 31 (3): 430–6. doi:10.1016/j.nut.2014.10.006. PMID 25701330.
External links
- Online Mendelian Inheritance in Man (OMIM) 105400 (ALS)
- The ALS Online Database
- A short but substantive overview of SOD and its literature.
- Damage-Based Theories of Aging Includes a discussion of the roles of SOD1 and SOD2 in aging.
- Physicians' Comm. For Responsible Med.
- SOD and Oxidative Stress Pathway Image
- Historical information on SOD research"The evolution of Free Radical Biology & Medicine: A 20-year history" and "Free Radical Biology & Medicine The last 20 years: The most highly cited papers"
- JM McCord discusses the discovery of SOD