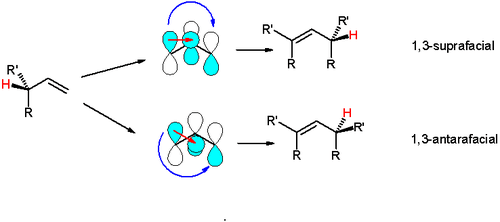
Antarafacial and suprafacial
Antarafacial and suprafacial are two topological concepts in organic chemistry describing the relationship between two simultaneous chemical bond making and/or bond breaking processes in or around a reaction center.[1] The reaction center can be a p-orbital, a conjugated system or even a sigma bond.
- This relationship is antarafacial when opposite faces are involved (think anti).
- It is suprafacial when both changes occur at the same face.
Many sigmatropic reactions and cycloadditions can be either suprafacial or antarafacial, and this determines the stereochemistry.
An example is the [1,3]-hydride shift, in which the interacting frontier orbitals are the allyl free radical and the hydrogen 1s orbitals. The suprafacial shift is symmetry-forbidden because orbitals with opposite algebraic signs overlap. The symmetry allowed antarafacial shift would require a strained transition state and is also unlikely. In contrast a symmetry allowed and suprafacial [1,5]-hydride shift is a common event.[2]
References
- ↑ IUPAC Gold Book definition Link
- ↑ F.A. Carey, R.J. Sundberg, Advanced Organic Chemistry Part A ISBN 0-306-41087-7