Thallium(I) bromide
-bromide-3D-SF.png) | |
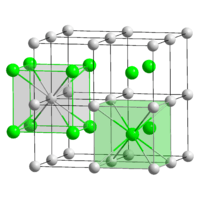 | |
| Names | |
|---|---|
| Other names
Thallium monobromide | |
| Identifiers | |
| 7789-40-4 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 56428 |
| ECHA InfoCard | 100.029.239 |
| EC Number | 232-163-0 |
| PubChem | 62677 |
| |
| |
| Properties | |
| TlBr | |
| Molar mass | 284.288 g/mol |
| Appearance | yellow-white crystalline solid |
| Density | 7.557 g/cm3 |
| Melting point | 480 °C (896 °F; 753 K) |
| Boiling point | 815 °C (1,499 °F; 1,088 K) |
| 0.047 g/100 mL (20 °C) | |
| Hazards | |
| EU classification (DSD) |
Very toxic (T+) Dangerous for the environment (N) |
| R-phrases | R26/28, R33, R51/53 |
| S-phrases | (S1/2), S13, S28, S45, S61 |
| Related compounds | |
| Other anions |
Thallium(I) fluoride, Thallium(I) chloride, Thallium(I) iodide |
| Other cations |
Indium(I) bromide, Lead(II) bromide Bismuth bromide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Thallium(I) bromide (TlBr), a chemical compound, available in an ultra-pure state is a compound semiconductor; used in room temperature X- and gamma-ray detectors and blue sensitive photodetectors; used as a real-time x-ray image sensor; also used as a standard for elemental thallium.
The crystalline structure is of cubic CsCl type at room temperature, but it lowers to the orthorombic thallium iodide type upon cooling, the transition temperature being likely affected by the impurities.[1][2][3]
A Material safety data sheet (MSDS) is available at http://www.espimetals.com/msds's/thalliumbromide.pdf. Thallium is extremely toxic and a cumulative poison which can be absorbed through the skin. Acute and chronic effects include fatigue, limb pain, peripheral neuritis, joint pain, loss of hair, Central nervous System effects, diarrhea, vomiting, liver and kidney damage.
References
- ↑ M Blackman et al "The Polymorphism of Thallium and Other Halides at Low Temperatures" Proc. Phys. Soc. 77 (1961) 471
- ↑ A-V Mudring "Thallium Halides - New Aspects of the Stereochemical Activity of Electron Lone Pairs of Heavier Main-Group Elements" Eur. J. Inorg. Chem. 6 (2007) 882
- ↑ R. P. Lowndes and C. H. Perry "Molecular structure and anharmonicity in thallium iodide" J. Chem. Phys. 58, 271 (1973)