Thallium(I) chloride
| |||
_chloride.jpg) | |||
| Names | |||
|---|---|---|---|
| IUPAC names
Thallium monochloride Thallium(I) chloride | |||
| Other names
Thallous chloride | |||
| Identifiers | |||
| 7791-12-0 | |||
| 3D model (Jmol) | Interactive image | ||
| ChEBI | CHEBI:37117 | ||
| ChemSpider | 23044 | ||
| ECHA InfoCard | 100.029.311 | ||
| PubChem | 24642 | ||
| |||
| |||
| Properties | |||
| TlCl | |||
| Molar mass | 239.82 g/mol | ||
| Appearance | white, odorless crystalline solid | ||
| Density | 7.004 g/cm3 (20 °C) | ||
| Melting point | 430 °C (806 °F; 703 K) | ||
| Boiling point | 720 °C (1,328 °F; 993 K) (decomposes) | ||
| 0.29 g/100 mL (15.5 °C) 0.318 g/100 mL (20 °C) 2.41 g/100 mL (100 °C) [1] | |||
| Solubility | insoluble in alcohol, acetone, NH4OH | ||
| Refractive index (nD) |
2.247 | ||
| Hazards | |||
| Safety data sheet | http://www.crystran.co.uk/uploads/files/178.pdf | ||
| EU classification (DSD) |
Very toxic (T+) Dangerous for the environment (N) | ||
| R-phrases | R26/28, R33, R51/53 | ||
| S-phrases | (S1/2), S13, S28, S45, S61 | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) |
24 mg/kg, oral, mouse | ||
| Related compounds | |||
| Other anions |
Thallium(I) fluoride Thallium(I) bromide Thallium(I) iodide | ||
| Other cations |
Thallium(III) chloride Silver(I) chloride Lead(II) chloride | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
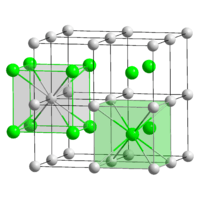
Thallium(I) chloride, also known as thallous chloride, is a chemical compound with the formula TlCl. This colourless solid is an intermediate in the isolation of thallium from its ores. Typically, an acidic solution of thallium(I) sulfate is treated with hydrochloric acid to precipitate insoluble thallium(I) chloride. This solid crystallizes in the caesium chloride motif.[2]
The low solubility of TlCl is exploited in chemical synthesis: treatment of metal chloride complexes with TlPF6, gives the corresponding metal hexafluorophosphate derivative. The resulting TlCl precipitate is separated by filtration of the reaction mixture. The overall methodology is similar to the use of AgPF6, except that Tl+ is much less oxidizing.
The crystalline structure is of cubic caesium chloride type at room temperature, but it lowers to the orthorhombic thallium iodide type upon cooling, the transition temperature being likely affected by the impurities.[3][4][5]
A very rare mineral lafossaite, Tl(Cl,Br), is a natural form of thallium(I) chloride.[6]
Thallium(I) chloride, like all thallium compounds, is highly toxic, although its low solubility limits its toxicity to a degree.
A radioactive isotope, thallium-201 chloride, also known as thallous chloride Tl 201, is used as a tracer in medical imaging.
References
- ↑ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- ↑ Holleman, A. F.; Wiberg, E. Inorganic Chemistry. Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ↑ M Blackman et al "The Polymorphism of Thallium and Other Halides at Low Temperatures" Proc. Phys. Soc. 77 (1961) 471
- ↑ A-V Mudring "Thallium Halides – New Aspects of the Stereochemical Activity of Electron Lone Pairs of Heavier Main-Group Elements" Eur. J. Inorg. Chem. 6 (2007) 882
- ↑ R. P. Lowndes and C. H. Perry "Molecular structure and anharmonicity in thallium iodide" J. Chem. Phys. 58, 271 (1973)
- ↑ http://www.mindat.org/min-27521.html
| Wikimedia Commons has media related to Thallium(I) chloride. |
-chloride-3D-SF-C.png)