Thermoelectric materials
| Thermoelectric effect |
|---|
 |
|
Principles
|
Thermoelectric materials show the thermoelectric effect in a strong or convenient form.
The thermoelectric effect refers to phenomena by which either a temperature difference creates an electric potential or an electric potential creates a temperature difference. These phenomena are known more specifically as the Seebeck effect (converting temperature to current), Peltier effect (converting current to temperature), and Thomson effect (conductor heating/cooling). While all materials have a nonzero thermoelectric effect, in most materials it is too small to be useful. However, low-cost materials that have a sufficiently strong thermoelectric effect (and other required properties) could be used in applications including power generation and refrigeration. A commonly used thermoelectric material in such applications is bismuth telluride (Bi
2Te
3).
Thermoelectric materials are used in thermoelectric systems for cooling or heating in niche applications,[1] and are being studied as a way to regenerate electricity from waste heat.[2]
Materials selection criteria
The usefulness of a material in thermoelectric systems is determined by the two factors device efficiency and power factor. These are determined by the material's electrical conductivity, thermal conductivity, Seebeck coefficient and behavior under changing temperatures.
Device efficiency
The efficiency of a thermoelectric device for electricity generation is given by , defined as
The ability of a given material to efficiently produce thermoelectric power is related to its dimensionless figure of merit given by:
- ,
which depends on the Seebeck coefficient S, thermal conductivity λ, electrical conductivity σ, and temperature T.
In an actual thermoelectric device, two materials are used. The maximum efficiency is then given by
where is the temperature at the hot junction and is the temperature at the surface being cooled. is the modified dimensionless figure of merit, which takes into consideration the thermoelectric capacity of both thermoelectric materials being used in the device and, after geometrical optimization regarding the legs sections,[3] is defined as
where is the electrical resistivity, is the average temperature between the hot and cold surfaces and the subscripts n and p denote properties related to the n- and p-type semiconducting thermoelectric materials, respectively. Since thermoelectric devices are heat engines, their efficiency is limited by the Carnot efficiency, hence the and terms in . Regardless, the coefficient of performance of current commercial thermoelectric refrigerators ranges from 0.3 to 0.6, one-sixth the value of traditional vapor-compression refrigerators.[4]
Power factor
In order to determine the usefulness of a material in a thermoelectric generator or a thermoelectric cooler the power factor is calculated by its Seebeck coefficient and its electrical conductivity under a given temperature difference:
where S is the Seebeck coefficient, and σ is the electrical conductivity.
Materials with a high power factor are able to 'generate' more energy (move more heat or extract more energy from that temperature difference) in a space-constrained application, but are not necessarily more efficient in generating this energy.
Aspects of materials choice
For good efficiency, materials with high electrical conductivity, low thermal conductivity and high Seebeck coefficient are needed.
State density: metals vs semiconductors
The band structure of semiconductors offers better thermoelectric effects than the band structure of metals.
The Fermi energy is below the conduction band causing the state density to be asymmetric around the Fermi energy. Therefore, the average electron energy of the conduction band is higher than the Fermi energy, making the system conducive for charge motion into a lower energy state. By contrast, the Fermi energy lies in the conduction band in metals. This makes the state density symmetric about the Fermi energy so that the average conduction electron energy is close to the Fermi energy, reducing the forces pushing for charge transport. Therefore, semiconductors are ideal thermoelectric materials.[5] Due to the small Seebeck coefficient metals have a very limited performance and the main materials of interest are Semiconductors.
Conductivity
In the efficiency equations above, thermal conductivity and electrical conductivity compete.
The thermal conductivity κ has mainly two components:
- κ = κ electron + κ phonon
According to the Wiedemann–Franz law, the higher the electrical conductivity, the higher κ electron becomes.[5] Thus in metals the ratio of thermal to electrical conductivity is about fixed, as the electron part dominates. In semiconductors, the phonon part is important and can not be neglect. It reduces the efficiency. For good efficiency a low ratio of κ phonon / κ electron is desired.
Therefore, it is necessary to minimize κ phonon and keep the electrical conductivity high. Thus semiconductors should be highly doped.
G. A. Slack[6] proposed that in order to optimize the figure of merit, phonons, which are responsible for thermal conductivity must experience the material as they would in a glass (experiencing a high degree of phonon scattering—lowering thermal conductivity) while electrons must experience it as a crystal (experiencing very little scattering—maintaining electrical conductivity). The figure of merit can be improved through the independent adjustment of these properties.
Quality Factor (detailed theory on semiconductors)
The maximum of a material is given by the material's Quality Factor:
where is the Boltzmann constant, is the reduced Planck constant, is the number of degenerated valleys for the band, is the average longitudinal elastic moduli, is the inertial effective mass, is the deformation potential coefficient, is the lattice thermal conduction, and is temperature. The figure of merit, , depends on doping concentration and temperature of the material of interest.[7] The material Quality Factor: is useful because it allows for an intrinsic comparisons of possible efficiency between different materials.[8] This relation shows that improving the electronic component , which primarily affects the Seebeck coefficient, will increase the quality factor of a material. A large density of states can be created due to a large number of conducting bands () or by flat bands giving a high band effective mass (). For isotropic materials . Therefore, it is desirable for thermoelectric materials to have high valley degeneracy in a very sharp band structure.[9]
Materials of interest
Strategies to improve thermoelectrics include both advanced bulk materials and the use of low-dimensional systems. Such approaches to reduce lattice thermal conductivity fall under three general material types: (1) Alloys: create point defects, vacancies, or rattling structures (heavy-ion species with large vibrational amplitudes contained within partially filled structural sites) to scatter phonons within the unit cell crystal;[10] (2) Complex crystals: separate the phonon glass from the electron crystal using approaches similar to those for superconductors (the region responsible for electron transport should be an electron crystal of a high-mobility semiconductor, while the phonon glass should ideally house disordered structures and dopants without disrupting the electron crystal, analogous to the charge reservoir in high-Tc superconductors[11]); (3) Multiphase nanocomposites: scatter phonons at the interfaces of nanostructured materials,[12] be they mixed composites or thin film superlattices.
Materials under consideration for thermoelectric device applications include:
Bismuth chalcogenides and their nanostructures
Materials such as Bi
2Te
3 and Bi
2Se
3 comprise some of the best performing room temperature thermoelectrics with a temperature-independent figure-of-merit, ZT, between 0.8 and 1.0.[13] Nanostructuring these materials to produce a layered superlattice structure of alternating Bi
2Te
3 and Sb
2Te
3 layers produces a device within which there is good electrical conductivity but perpendicular to which thermal conductivity is poor. The result is an enhanced ZT (approximately 2.4 at room temperature for p-type).[14] Note that this high value of ZT has not been independently confirmed due to the complicated demands on the growth of such superlattices and device fabrication; however the material ZT values are consistent with the performance of hot-spot coolers made out of these materials and validated at Intel Labs.
Bismuth telluride and its solid solutions are good thermoelectric materials at room temperature and therefore suitable for refrigeration applications around 300 K. The Czochralski method has been used to grow single crystalline bismuth telluride compounds. These compounds are usually obtained with directional solidification from melt or powder metallurgy processes. Materials produced with these methods have lower efficiency than single crystalline ones due to the random orientation of crystal grains, but their mechanical properties are superior and the sensitivity to structural defects and impurities is lower due to high optimal carrier concentration.
The required carrier concentration is obtained by choosing a nonstoichiometric composition, which is achieved by introducing excess bismuth or tellurium atoms to primary melt or by dopant impurities. Some possible dopants are halogens and group IV and V atoms. Due to the small bandgap (0.16 eV) Bi2Te3 is partially degenerate and the corresponding Fermi-level should be close to the conduction band minimum at room temperature. The size of the band-gap means that Bi2Te3 has high intrinsic carrier concentration. Therefore, minority carrier conduction cannot be neglected for small stoichiometric deviations. Use of telluride compounds is limited by the toxicity and rarity of tellurium.[15]
Lead telluride
In 2008 Joseph Heremans and his colleagues demonstrated that thallium-doped lead telluride alloy (PbTe) achieves a ZT of 1.5 at 773 K.[16] Later, Snyder and his colleagues reported ZT~1.4 at 750 K in sodium-doped PbTe,[17] and ZT~1.8 at 850 K in sodium-doped PbTe1-xSex alloy.[18] Snyder’s group determined that both thallium and sodium alter the electronic structure of the crystal increasing electric conductivity. They also claim that selenium increases electric conductivity and reduces thermal conductivity.
In 2012 another team used lead telluride to convert 15 to 20 percent of waste heat to electricity, reaching a ZT of 2.2, which they claimed was the highest yet reported.[19][20]
Inorganic clathrates
Inorganic clathrates have the general formula AxByC46-y (type I) and AxByC136-y (type II), where B and C are group III and IV elements, respectively, which form the framework where “guest” A atoms (alkali or alkaline earth metal) are encapsulated in two different polyhedra facing each other. The differences between types I and II come from the number and size of voids present in their unit cells. Transport properties depend on the framework's properties, but tuning is possible by changing the “guest” atoms.[21][22]
The most direct approach to synthesize and optimize the thermoelectric properties of semiconducting type I clathrates is substitutional doping, where some framework atoms are replaced with dopant atoms. In addition, powder metallurgical and crystal growth techniques have been used in clathrate synthesis. The structural and chemical properties of clathrates enable the optimization of their transport properties as a function of stoichiometry. The structure of type II materials allows a partial filling of the polyhedra, enabling better tuning of the electrical properties and therefore better control of the doping level. Partially filled variants can be synthesized as semiconducting or even insulating.
Blake et al. have predicted ZT~0.5 at room temperature and ZT~1.7 at 800 K for optimized compositions. Kuznetsov et al. measured electrical resistance and Seebeck coefficient for three different type I clathrates above room temperature and by estimating high temperature thermal conductivity from the published low temperature data they obtained ZT~0.7 at 700 K for Ba8Ga16Ge30 and ZT~0.87 at 870 K for Ba8Ga16Si30.[23]
Magnesium group IV compounds
Mg2BIV (BIV=Si, Ge, Sn) compounds and their solid solutions are good thermoelectric materials and their ZT values are comparable with those of established materials. Due to a lack of systematic studies about their thermoelectric properties, however, the suitability of these materials and in particular their quasi-ternary solutions, for thermoelectric energy conversion remains in question. The appropriate production methods are based on direct co-melting, but mechanical alloying has also been used. During synthesis, magnesium losses due to evaporation and segregation of components (especially for Mg2Sn) need to be taken into account. Directed crystallization methods can produce single crystalline material. Solid solutions and doped compounds have to be annealed in order to produce homogeneous samples - with the same properties throughout. At 800 K Mg2Si1-xSnx has been reported to have a figure of merit about 0.9.[24]
Silicides
Higher silicides display ZT levels with current materials. They are mechanically and chemically strong and therefore can often be used in harsh environments without protection. Possible fabrication methods include Czochralski and floating zone for single crystals and hot pressing and sintering for polycrystalline.[25]
Skutterudite thermoelectrics
Recently, skutterudite materials have sparked the interest of researchers in search of new thermoelectrics.[26] These structures are of the form (Co,Ni,Fe)(P,Sb,As)
3 and are cubic with space group Im3. Unfilled, these materials contain voids into which low-coordination ions (usually rare earth elements) can be inserted in order to alter thermal conductivity by producing sources for lattice phonon scattering and decrease thermal conductivity due to the lattice without reducing electrical conductivity.[27] Such qualities make these materials exhibit PGEC behavior.
Skutterudites have the chemical formula LM4X12, where L is a rare earth metal, M a transition metal and X a metalloid, a group V element or pnictogen whose properties lie between those of a metal and nonmetal such as phosphorus, antimony, or arsenic. These materials could be potential in multistage thermoelectric devices as it has been shown that they have ZT>1.0, but their properties are not well known.[28]
Oxide thermoelectrics
Their layered superlattice structure gives homologous oxide compounds (such as those of the form (SrTiO
3)n(SrO)
m—the Ruddleson-Popper phase) potential in high-temperature thermoelectric devices.[29] These materials exhibit low thermal conductivity perpendicular to the layers while maintaining electrical conductivity within the layers. ZT is relatively low (~0.34 at 1,000K),[30] but their enhanced thermal stability, as compared to conventional high-ZT bismuth compounds, makes them superior for use in high-temperature applications.[31]
Interest in oxides as thermoelectric materials was reawakened in 1997 when NaxCoO2 was found to exhibit good thermoelectric behavior. In addition to their thermal stability, other advantages of oxides are their nontoxicity and high oxidation resistance. Simultaneously controlling both the electric and phonon systems may require nanostructured materials. Some layered oxide materials are thought to have ZT~2.7 at 900 K. If the layers in a given material have the same stoichiometry, they will be stacked so that the same atoms will not be positioned on top of each other, impeding phonon conductivity perpendicular to the layers.[29] Recently, oxide thermoelectrics have gained a lot of attention so that the range of promising phases increased drastically. Novel members of this family include ZnO,[32][33] MnO2,[34][35] and NbO2,[36][37] to name but a few.
Half Heusler alloys
Half Heusler alloys have potential for high temperature power generation applications especially as n-type material. These alloys have three components that originate from different element groups or combinations of elements. Two of the groups are composed of transition metals and the third group consists of metals and metalloids. Currently only n-type material is usable in thermoelectrics but some sources claim that they have achieved ZT~1.5 at 700 K, but according to other sources only ZT~0.5 at 700 K has been achieved. They state that primary reason for this difference is the disagreement between thermal conductivities measured by different groups. These alloys are relatively cheap and also have a high power factor.[38]
Electrically conducting organic materials
Some electrically conducting organic materials may have a higher figure of merit than existing inorganic materials. Seebeck coefficient can be even millivolts per Kelvin but electrical conductivity is usually low, resulting in small ZT values. Quasi-one-dimensional organic crystals are formed from linear chains or stacks of molecules that are packed into a 3D crystal. Under certain conditions some Q1D organic crystals may have ZT~20 at room temperature for both p- and n-type materials. This has been credited to an unspecified interference between two main electron-phonon interactions leading to the formation of narrow strip of states in the conduction band with a significantly reduced scattering rate as the mechanism compensate each other, yielding high ZT.[39]
Silicon-germanium
Silicon-germanium alloys are currently the best thermoelectric materials around 1000 ℃ and are therefore used in some radioisotope thermoelectric generators (RTG) (notably the MHW-RTG and GPHS-RTG) and some other high temperature applications, such as waste heat recovery. Usability of silicon-germanium alloys is limited by their price and mid-range ZT (~0.7).
Sodium cobaltate
Experiments on crystals of sodium cobaltate, using X-ray and neutron scattering experiments carried out at the European Synchrotron Radiation Facility (ESRF) and the Institut Laue-Langevin (ILL) in Grenoble were able to suppress thermal conductivity by a factor of six compared to vacancy-free sodium cobaltate. The experiments agreed with corresponding density functional calculations. The technique involved large anharmonic displacements of Na
0.8CoO
2 contained within the crystals.[40][41]
Amorphous materials
In 2002, Nolas and Goldsmid have come up with a suggestion that systems with the phonon mean free path larger than the charge carrier mean free path can exhibit an enhanced thermoelectric efficiency.[42] This can be realized in amorphous thermoelectrics and soon they became a focus of many studies. This ground-breaking idea was accomplished in Cu-Ge-Te,[43] NbO2,[44] In-Ga-Zn-O,[45] Zr-Ni-Sn,[46] Si-Au,[47] and Ti-Pb-V-O[48] amorphous systems. It should be mentioned that modelling of transport properties is challenging enough without breaking the long-range order so that design of amorphous thermoelectrics is at its infancy. Naturally, amorphous thermoelectrics give rise to extensive phonon scattering, which is still a challenge for crystalline thermoelectrics. A bright future is expected for these materials.
Functionally graded materials
Functionally graded materials make it possible to improve the conversion efficiency of existing thermoelectrics. These materials have a non-uniform carrier concentration distribution and in some cases also solid solution composition. In power generation applications the temperature difference can be several hundred degrees and therefore devices made from homogeneous materials have some part that operates at the temperature where ZT is substantially lower than its maximum value. This problem can be solved by using materials whose transport properties vary along their length thus enabling substantial improvements to the operating efficiency over large temperature differences. This is possible with functionally graded materials as they have a variable carrier concentration along the length of the material, which is optimized for operations over specific temperature range.[49]
Nanomaterials and superlattices
In addition to nanostructured Bi
2Te
3/Sb
2Te
3 superlattice thin films, other nanomaterials show potential in improving thermoelectric properties.
PbTe/PbSeTe quantum dot superlattice
Another example of a superlattice involves a PbTe/PbSeTe quantum dot superlattices provides an enhanced ZT (approximately 1.5 at room temperature) that was higher than the bulk ZT value for either PbTe or PbSeTe (approximately 0.5).[50]
Nanocrystal stability and thermal conductivity
Not all nanocrystalline materials are stable, because the crystal size can grow at high temperatures, ruining the materials' desired characteristics.
Nanocrystalline materials have many interfaces between crystals, which Physics of SASER scatter phonons so the thermal conductivity is reduced. Phonons are confined to the grain, if their mean free path is larger than the material grain size.
Measured lattice thermal conductivity in nanowires is known to depend on roughness, the method of synthesis and properties of the source material.[51]
Nanocrystalline transition metal silicides
Nanocrystalline transition metal silicides are a promising material group for thermoelectric applications, because they fulfill several criteria that are demanded from the commercial applications point of view. In some nanocrystalline transition metal silicides the power factor is higher than in the corresponding polycrystalline material but the lack of reliable data on thermal conductivity prevents the evaluation of their thermoelectric efficiency.[52]
Nanostructured skutterudites
Skutterudites, a cobalt arsenide mineral with variable amounts of nickel and iron, can be produced artificially, and are candidates for better thermoelectric materials.
One advantage of nanostructured skutterudites over normal skutterudites is their reduced thermal conductivity, caused by grain boundary scattering. ZT values of ~0.65 and > 0.4 have been achieved with CoSb3 based samples; the former values were 2.0 for Ni and 0.75 for Te-doped material at 680 K and latter for Au-composite at T > 700 K.[53]
Even greater performance improvements can be achieved by using composites and by controlling the grain size, the compaction conditions of polycrystalline samples and the carrier concentration.
Graphene
Due to the unique nature of graphene, it is possible to develop a thermoelectric device based on it with an extremely high Seebeck coefficient.
One theoretical study suggests that the Seebeck coefficient might achieve a value of 30 mV/K at room temperature and ZT for their proposed device would be approximately 20.[54]
Superlattices and roughness
Superlattices - nano structured thermocouples, are considered a good candidate for better thermoelectric device manufacturing, with materials that can be used in manufacturing this structure.
Their production is expensive for general-use due to fabrication processes based on expensive thin-film growth methods. However, since the amount of thin-film materials required for device fabrication with superlattices, is so much less than thin-film materials in bulk thermoelectric materials (almost by a factor of 1/10,000) the long-term cost advantage is indeed favorable.
This is particularly true given the limited availability of tellurium causing competing solar applications for thermoelectric coupling systems to rise.
Superlattice structures also allow the independent manipulation of transport parameters by adjusting the structure itself, enabling research for better understanding of the thermoelectric phenomena in nanoscale, and studying the phonon-blocking electron-transmitting structures - explaining the changes in electric field and conductivity due to the material's nano-structure.[14]
Many strategies exist to decrease the superlattice thermal conductivity that are based on engineering of phonon transport. The thermal conductivity along the film plane and wire axis can be reduced by creating diffuse interface scattering and by reducing the interface separation distance, both which are caused by interface roughness.
Interface roughness can naturally occur or may be artificially induced.
In nature, roughness is caused by the mixing of atoms of foreign elements. Artificial roughness can be created using various structure types, such as quantum dot interfaces and thin-films on step-covered substrates.[55]
Problems in superlattices
Reduced electrical conductivity:
Reduced phonon-scattering interface structures often also exhibit a decrease in electrical conductivity.
The thermal conductivity in the cross-plane direction of the lattice is usually very low, but depending on the type of superlattice, the thermoelectric coefficient may increase because of changes to the band structure.
Low thermal conductivity in superlattices is usually due to strong interface scattering of phonons. Minibands are caused by the lack of quantum confinement within a well. The mini-band structure depends on the superlattice period so that with a very short period (~1 nm) the band structure approaches the alloy limit and with a long period (≥ ~60 nm) minibands become so close to each other that they can be approximated with a continuum.[56]
Superlatice structure countermeasures:
Counter measures can be taken which practically eliminate the problem of decreased electrical conductivity in a reduced phonon-scattering interface. These measures include the proper choice of superlattice structure, taking advantage of mini-band conduction across superlattices, and avoiding quantum-confinement. It has been shown that because electrons and phonons have different wavelengths, it is possible to engineer the structure in such a way that phonons are scattered more diffusely at the interface than electrons.[14]
Phonon confinement countermeasures:
Another approach to overcome the decrease in electrical conductivity in reduced phonon-scattering structures is to increase phonon reflectivity and therefore decrease the thermal conductivity perpendicular to the interfaces.
This can be achieved by increasing the mismatch between the materials in adjacent layers, including density, group velocity, specific heat, and the phonon-spectrum.
Interface roughness causes diffuse phonon scattering, which either increases or decreases the phonon reflectivity at the interfaces. A mismatch between bulk dispersion relations confines phonons, and the confinement becomes more favorable as the difference in dispersion increases.
The amount of confinement is currently unknown as only some models and experimental data exist. As with a previous method, the effects on the electrical conductivity have to be considered.[55]
Attempts to Localize long wavelength phonons by aperiodic superlattices or composite superlattices with different periodicities have been made. In addition, defects, especially dislocations, can be used to reduce thermal conductivity in low dimensional systems.[55]
Parasitic heat:
Parasitic heat conduction in the barrier layers could cause significant performance loss. It has been proposed but not tested that this can be overcome by choosing a certain correct distance between the quantum wells.
The Seebeck coefficient can change its sign in superlattice nanowires due to the existence of minigaps as Fermi energy varies. This indicates that superlattices can be tailored to exhibit n or p-type behavior by using the same dopants as those that are used for corresponding bulk materials by carefully controlling Fermi energy or the dopant concentration. With nanowire arrays, it is possible to exploit semimetal-semiconductor transition due to the quantum confinement and use materials that normally would not be good thermoelectric materials in bulk form. Such elements are for example bismuth. The Seebeck effect could also be used to determine the carrier concentration and Fermi energy in nanowires.[57]
In quantum dot thermoelectrics, unconventional or nonband transport behavior (e.g. tunneling or hopping) is necessary to utilize their special electronic band structure in the transport direction. It is possible to achieve ZT>2 at elevated temperatures with quantum dot superlattices, but they are almost always unsuitable for mass production.
However, in superlattices, where quantum-effects are not involved, with film thickness of only a few micrometers (µm) to about 15 µm, Bi2Te3/Sb2Te3 superlattice material has been made into high-performance microcoolers and other devices. The performance of hot-spot coolers[14] are consistent with the reported ZT~2.4 of superlattice materials at 300 K.[58]
Nanocomposites are promising material class for bulk thermoelectric devices, but several challenges have to be overcome to make them suitable for practical applications. It is not well understood why the improved thermoelectric properties appear only in certain materials with specific fabrication processes.[59]
SrTe nanocrystals can be embedded in a bulk PbTe matrix so that rocksalt lattices of both materials are completely aligned (endotaxy) with optimal molar concentration for SrTe only 2%. This can cause strong phonon scattering but would not affect charge transport. In such case, ZT~1.7 can be achieved at 815 K for p-type material.[60]
Tin selenide
In 2014 a research group discovered that tin selenide (SnSe) has a ZT of 2.6 along the b axis of the unit cell.[61][62] This is the highest value reported to date. This high ZT figure of merit has been attributed to an extremely low thermal conductivity found in the SnSe lattice. Specifically, SnSe demonstrated a lattice thermal conductivity of 0.23 W·m−1·K−1, which is much lower than previously reported values of 0.5 W·m−1·K−1 and greater.[63] This SnSe material also exhibited a ZT of 2.3±0.3 along the c-axis and 0.8±0.2 along the a-axis. These excellent figures of merit were obtained by researchers working at elevated temperatures, specifically 923 K (650 °C). As shown by the figures below, SnSe performance metrics were found to significantly improve at higher temperatures; this is due to a structural change that is discussed below. Power factor, conductivity, and thermal conductivity all reach their optimal values at or above 750 K, and appear to plateau at higher temperatures.
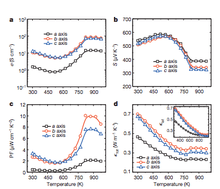
Although it exists at room temperature in an orthorhombic structure with space group Pnma, SnSe has been shown to undergo a transition to a structure with higher symmetry, space group Cmcm, at higher temperatures.[64] This structure consists of Sn-Se planes that are stacked upwards in the a-direction, which accounts for the poor performance out-of-plane (along a-axis). Upon transitioning to the Cmcm structure, SnSe maintains its low thermal conductivity but exhibits higher carrier mobilities, leading to its excellent ZT value.[63]
One particular impediment to further development of SnSe is that it has a relatively low carrier concentration: approximately 1017 cm−3. Further compounding this issue is the fact that SnSe has been reported to have low doping efficiency.[65]
However, such single crystalline materials suffer from inability to make useful devices due to their brittleness as well as narrow range of temperatures, where ZT is reported to be high. Further, polycrystalline materials made out of these compounds by several investigators have not confirmed the high ZT of these materials.
Production methods
Production methods for these materials can be divided into powder and crystal growth based techniques. Powder based techniques offer excellent ability to control and maintain desired carrier distribution. In crystal growth techniques dopants are often mixed with melt, but diffusion from gaseous phase can also be used. In the zone melting techniques disks of different materials are stacked on top of others and then materials are mixed with each other when a traveling heater causes melting. In powder techniques, either different powders are mixed with a varying ratio before melting or they are in different layers as a stack before pressing and melting.
There are applications, such as cooling of electronic circuits, where thin films are required. Therefore, thermoelectric materials can also be synthesized using physical vapor deposition techniques. Another reason to utilize these methods is to design these phases and provide guidance for bulk applications.
Applications
Refrigeration
Thermoelectric materials can be used as refrigerators, called "thermoelectric coolers", or "Peltier coolers" after the Peltier effect that controls their operation. As a refrigeration technology, Peltier cooling is far less common than vapor-compression refrigeration. The main advantages of a Peltier cooler (compared to a vapor-compression refrigerator) are its lack of moving parts or refrigerant, and its small size and flexible shape (form factor).
The main disadvantage of Peltier coolers is low efficiency. It is estimated that materials with ZT>3 (about 20–30% Carnot efficiency) would be required to replace traditional coolers in most applications.[50] Today, Peltier coolers are only used in niche applications, especially small scale, where efficiency is not important.
Power generation
Thermoelectric efficiency depends on the figure of merit, ZT. There is no theoretical upper limit to ZT, and as ZT approaches infinity, the thermoelectric efficiency approaches the Carnot limit. However, no known thermoelectrics have a ZT>3.[66] As of 2010, thermoelectric generators serve application niches where efficiency and cost are less important than reliability, light weight, and small size.[67]
Internal combustion engines capture 20–25% of the energy released during fuel combustion.[68] Increasing the conversion rate can increase mileage and provide more electricity for on-board controls and creature comforts (stability controls, telematics, navigation systems, electronic braking, etc.)[69] It may be possible to shift energy draw from the engine (in certain cases) to the electrical load in the car, e.g. electrical power steering or electrical coolant pump operation.[68]
Cogeneration power plants use the heat produced during electricity generation for alternative purposes. Thermoelectrics may find applications in such systems or in solar thermal energy generation.[70]
See also
References
- ↑ Saving Energy and Reducing CO2 Emissions with Electricity, Clark W. Gellings, page 176. ISBN 0-88173-6678
- ↑ Weak electron–phonon coupling contributing to high thermoelectric performance in n-type PbSe Study on thermoelectric coupling, Proceedings of the National Academy of Sciences USA, Jun 19, 2012; 109(25) (National Center for Biotechnology Information website)
- ↑ A.F. Ioffe, Physics of semiconductors, Academic Press Inc., New York (1960)
- ↑ Solar refrigeration options – a state-of-the-art review. D.S. Kim, C.A. Infante Ferreira. 2008, International Journal of Refrigeration, pp. 3–15. DOI web link
- 1 2 Timothy D. Sands (2005), Designing Nanocomposite Thermoelectric Materials
- ↑ Slack GA., in Rowe 2005
- ↑ Mahan, G. D. (1997-01-01). SPAEPEN, HENRY EHRENREICH and FRANS, ed. Good Thermoelectrics. 51. Academic Press. pp. 81–157.
- ↑ Koumoto, Kunihito; Mori, Takao (2013-07-20). Thermoelectric Nanomaterials: Materials Design and Applications. Springer Science & Business Media. ISBN 9783642375378.
- ↑ Yanzhong, Pei,; Heng, Wang,; J., Snyder, G. (2012-12-04). "Band Engineering of Thermoelectric Materials". authors.library.caltech.edu. Retrieved 2015-10-23.
- ↑ Bhandari, C. M. in Rowe 2005, pp. 55–65
- ↑ Cava, R. J. (1990). "Structural chemistry and the local charge picture of copper-oxide superconductors". Science. 247 (4943): 656–62. Bibcode:1990Sci...247..656C. doi:10.1126/science.247.4943.656. PMID 17771881.
- ↑ Dresselhaus, M. S.; Chen, G.; Tang, M. Y.; Yang, R. G.; Lee, H.; Wang, D. Z.; Ren, Z. F.; Fleurial, J.-P.; Gogna, P. (2007). "New directions for low-dimensional thermoelectric materials" (PDF). Advanced Materials. 19 (8): 1043. doi:10.1002/adma.200600527.
- ↑ Duck Young Chung; Hogan, T.; Schindler, J.; Iordarridis, L.; Brazis, P.; Kannewurf, C.R.; Baoxing Chen; Uher, C.; Kanatzidis, M.G. (1997). "XVI ICT '97. Proceedings ICT'97. 16th International Conference on Thermoelectrics (Cat. No.97TH8291)": 459. doi:10.1109/ICT.1997.667185. ISBN 0-7803-4057-4.
|chapter=ignored (help) - 1 2 3 4 Venkatasubramanian, Rama; Siivola, Edward; Colpitts, Thomas; O'Quinn, Brooks (2001). "Thin-film thermoelectric devices with high room-temperature figures of merit". Nature. 413 (6856): 597–602. Bibcode:2001Natur.413..597V. doi:10.1038/35098012. PMID 11595940.
- ↑ Rowe 2005, Chapter 27.
- ↑ Heremans, J. P.; Jovovic, V.; Toberer, E. S.; Saramat, A.; Kurosaki, K.; Charoenphakdee, A.; Yamanaka, S.; Snyder, G. J. (2008). "Enhancement of Thermoelectric Efficiency in PbTe by Distortion of the Electronic Density of States". Science. 321 (5888): 554–7. Bibcode:2008Sci...321..554H. doi:10.1126/science.1159725. PMID 18653890.
- ↑ Pei, Yanzhong; Lalonde, Aaron; Iwanaga, Shiho; Snyder, G. Jeffrey (2011). "High thermoelectric figure of merit in heavy hole dominated PbTe". Energy & Environmental Science. 4 (6): 2085. doi:10.1039/C0EE00456A.
- ↑ Pei, Yanzhong; Shi, Xiaoya; Lalonde, Aaron; Wang, Heng; Chen, Lidong; Snyder, G. Jeffrey (2011). "Convergence of electronic bands for high performance bulk thermoelectrics". Nature. 473 (7345): 66–9. Bibcode:2011Natur.473...66P. doi:10.1038/nature09996. PMID 21544143.
- ↑ Quick, Darren (September 20, 2012). "World's most efficient thermoelectric material developed". Gizmag. Retrieved December 2014. Check date values in:
|access-date=(help) - ↑ Biswas, K.; He, J.; Blum, I. D.; Wu, C. I.; Hogan, T. P.; Seidman, D. N.; Dravid, V. P.; Kanatzidis, M. G. (2012). "High-performance bulk thermoelectrics with all-scale hierarchical architectures". Nature. 489 (7416): 414. Bibcode:2012Natur.489..414B. doi:10.1038/nature11439.
- ↑ Rowe 2005, 32–33.
- ↑ Gatti, C., Bertini, L., Blake, N. P. and Iversen, B. B. (September 2003). "Guest–Framework Interaction in Type I Inorganic Clathrates with Promising Thermoelectric Properties: On the Ionic versus Neutral Nature of the Alkaline-Earth Metal Guest A in A8Ga16Ge30 (A=Sr, Ba)". Chemistry. 9 (18): 4556–68. doi:10.1002/chem.200304837. PMID 14502642.
- ↑ Rowe 2005, Chapter 32-33.
- ↑ Rowe 2005, Chapter 29.
- ↑ Rowe 2005, Chapter 31.
- ↑ Caillat, T., Borshchevsky, A., and Fleurial, J.-P., In Proceedings of 7th International Conference TEs, K. Rao, ed., pp. 98 – 101. University of Texas, Arlington, 1993.
- ↑ Nolas, G. S.; Slack, G. A.; Morelli, D. T.; Tritt, T. M.; Ehrlich, A. C. (1996). "The effect of rare-earth filling on the lattice thermal conductivity of skutterudites". Journal of Applied Physics. 79 (8): 4002. Bibcode:1996JAP....79.4002N. doi:10.1063/1.361828.
- ↑ Rowe 2005, Chapter 34.
- 1 2 Rowe 2005, Chapter 35.
- ↑ Wunderlich, W.; Ohta, S.; Ohta, H.; Koumoto, K. (2005). "Effective mass and thermoelectric properties of SrTiO3-based natural superlattices evaluated by ab-initio calculations": 252. doi:10.1109/ICT.2005.1519931. ISBN 0-7803-9552-2.
|chapter=ignored (help) - ↑ Senthilkumar, Meenakshisundaram; Vijayaraghavan, Rajagopalan (2009). "High-temperature resistivity and thermoelectric properties of coupled substituted Ca3Co2O6". Science and Technology of Advanced Materials (free download). 10: 015007. Bibcode:2009STAdM..10a5007S. doi:10.1088/1468-6996/10/1/015007.
- ↑ Ohtaki, M.; Tsubota, T.; Eguchi, K.; Arai, H. (1996). "High‐temperature thermoelectric properties of (Zn1−xAlx)O". Journal of Applied Physics. 79: 1816. doi:10.1063/1.360976.
- ↑ Ruoho, M.; Pale, V.; Erdmanis, M.; Tittonen, I. (2013). "Influence of aluminium doping on thermoelectric performance of atomic layer deposited ZnO thin films". Applied Physics Letters. 103: 203903. doi:10.1063/1.4831980.
- ↑ Walia, S.; Balendhran, S.; Yi, P.; Yao, D.; et al. (2013). "MnO2‑Based Thermopower Wave Sources with Exceptionally Large Output Voltages". Journal of Physical Chemistry C. 117: 9137. doi:10.1021/jp401731b.
- ↑ Music, D.; Schneider, J.M. (2015). "Critical evaluation of the colossal Seebeck coefficient of nanostructured rutile MnO2". Journal of Physics: Condensed Matter. 27: 115302. doi:10.1088/0953-8984/27/11/115302.
- ↑ Music, D.; Chen, Y.-T.; Bliem, P.; Geyer, R.W. (2015). "Amorphous-crystalline transition in thermoelectric NbO2". Journal of Physics D: Applied Physics. 48: 275301. doi:10.1088/0022-3727/48/27/275301.
- ↑ Onozato, T.; Katase, T.; Yamamoto, A.; et al. (2016). "Optoelectronic properties of valence-state-controlled amorphous niobium oxide". Journal of Physics: Condensed Matter. 28: 255001. doi:10.1088/0953-8984/28/25/255001.
- ↑ Culp, Slade R.; Poon, S. Joseph; Hickman, Nicoleta; Tritt, Terry M.; Blumm, J. (2006). "Effect of substitutions on the thermoelectric figure of merit of half-Heusler phases at 800 °C". Applied Physics Letters. 88 (4): 042106. Bibcode:2006ApPhL..88d2106C. doi:10.1063/1.2168019.
- ↑ Rowe 2005, Chapter 36.
- ↑ "Improved thermoelectric materials may give a push to Moore's law". KurzweilAI. Retrieved 2013-09-02.
- ↑ Voneshen, D. J.; Refson, K.; Borissenko, E.; Krisch, M.; Bosak, A.; Piovano, A.; Cemal, E.; Enderle, M.; Gutmann, M. J.; Hoesch, M.; Roger, M.; Gannon, L.; Boothroyd, A. T.; Uthayakumar, S.; Porter, D. G.; Goff, J. P. (2013). "Suppression of thermal conductivity by rattling modes in thermoelectric sodium cobaltate". Nature Materials. Bibcode:2013NatMa..12.1028V. doi:10.1038/nmat3739.
- ↑ Nolas, G.S.; Goldsmid, H.J. (2002). "The figure of merit in amorphous thermoelectrics". Physica Status Solidi A. 194: 271. doi:10.1002/1521-396X(200211)194:1<271::AID-PSSA271>3.0.CO;2-T.
- ↑ Goncalves, A.P.; Lopes, E.B.; Rouleau, O.; Godart, C. (2010). "Conducting glasses as new potential thermoelectric materials: the Cu-Ge-Te case". Journal of Materials Chemistry. 20: 1516. doi:10.1039/B908579C.
- ↑ Music, D.; Geyer, R.W.; Hans, M. (2016). "High-throughput exploration of thermoelectric and mechanical properties of amorphous NbO2 with transition metal additions". Journal of Applied Physics. 120: 045104. doi:10.1063/1.4959608.
- ↑ Fujimoto, Y.; Uenuma, M.; Ishikawa, Y.; Uraoka, Y. (2015). "Analysis of thermoelectric properties of amorphous InGaZnO thin film by controlling carrier concentration". AIP Advances. 5: 097209. doi:10.1063/1.4931951.
- ↑ Zhou, Y.; Tan, Q.; Zhu, J.; Li, S.; Liu, C.; Lei, Y.; Li, L. (2015). "Thermoelectric properties of amorphous Zr-Ni-Sn thin films deposited by magnetron sputtering". Journal of Electronic Materials. 44: 1957. doi:10.1007/s11664-014-3610-7.
- ↑ Takiguchi, H.; Yoshikawa, Z.; Miyazaki, H.; Okamoto (2010). "The role of Au in the thermoelectric properties of amorphous Ge/Au and Si/Au thin films". Journal of Electronic Materials. 39: 1627. doi:10.1007/s11664-010-1267-4.
|first5=missing|last5=in Authors list (help) - ↑ "DC electrical conductivity, thermoelectric power measurements of TiO2-substituted lead vanadate glasses". Physica B. 387: 45. 2007. doi:10.1016/j.physb.2006.03.026.
- ↑ Rowe 2005, Chapter 38.
- 1 2 Harman, T. C.; Taylor, PJ; Walsh, MP; Laforge, BE (2002). "Quantum dot superlattice thermoelectric materials and devices" (PDF). Science. 297 (5590): 2229–32. Bibcode:2002Sci...297.2229H. doi:10.1126/science.1072886. PMID 12351781.
- ↑ Akram I. Boukai, Yuri Bunimovich; Jamil Tahir-Kheli, Jen-Kan Yu; Tahir-Kheli, Jamil; Yu, Jen-Kan; Goddard Iii, William A.; Heath, James R. (2008). "Silicon nanowires as efficient thermoelectric materials". Nature letters. 451 (3): 19. Bibcode:2008Natur.451..168B. doi:10.1038/nature06458.
- ↑ Rowe 2005, Chapter 40.
- ↑ Rowe 2005, Chapter 41.
- ↑ Dragoman, D.; Dragoman, M. (2007). "Giant thermoelectric effect in graphene". Applied Physics Letters. 91 (20): 203116. Bibcode:2007ApPhL..91t3116D. doi:10.1063/1.2814080.
- 1 2 3 Chen, G.; Dresselhaus, M. S.; Dresselhaus, G.; Fleurial, J.-P.; Caillat, T. (2003). "Recent developments in thermoelectric materials". International Materials Reviews. 48: 45. doi:10.1179/095066003225010182.
- ↑ Rowe, ch. 16, 39
- ↑ Rowe 2005, Chapter 39.
- ↑ Rowe 2005, Chapter 49.
- ↑ Minnich, A. J.; Dresselhaus, M. S.; Ren, Z. F.; Chen, G. (2009). "Bulk nanostructured thermoelectric materials: current research and future prospects". Energy & Environmental Science. 2 (5): 466. doi:10.1039/b822664b.
- ↑ Biswas, Kanishka; He, Jiaqing; Zhang, Qichun; Wang, Guoyu; Uher, Ctirad; Dravid, Vinayak P.; Kanatzidis, Mercouri G. (2011). "Strained endotaxial nanostructure with high thermoelectric figure of merit". Nature Chemistry. 3 (2): 160–6. Bibcode:2011NatCh...3..160B. doi:10.1038/nchem.955. PMID 21258390.
- ↑ Zhao, Li-Dong; Lo, Shih-Han; Zhang, Yongsheng; Sun, Hui; Tan, Gangjian; Uher, Ctirad; Wolverton, C.; Dravid, Vinayak P.; Kanatzidis, Mercouri G. (2014). "Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals". Nature. 508 (7496): 373–7. Bibcode:2014Natur.508..373Z. doi:10.1038/nature13184. PMID 24740068.
- ↑ Zhang, H. and Talapin, D. V. (2014), Thermoelectric Tin Selenide: The Beauty of Simplicity. Angew. Chem. Int. Ed., 53: 9126–9127. doi:10.1002/anie.201405683
- 1 2 3 L-D. Zhao, S-H. Lo, Y. Zhang, H. Sun, G. Tan, C. Uher, C. Wolverton, V. Dravid, and M. Kanatzidis, “Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals,” Nature, 508, 373–377 (2014).
- ↑ A. C. Bernardes-Silva, A.F. Mesquita, E. de Moura Neto, A.O. Porto, J.D. Ardisson, G.M. de Lima, F.S. Lameiras, “XRD and 119Sn Mossbauer spectroscopy characterization of SnSe obtained from a simple chemical route,” Materials Research Bulletin, 40, 1497–1505 (2005).
- ↑ C-L. Chen, H. Wang, Y-Y. Chen, T. Daya and G. J. Snyder, “Thermoelectric properties of p-type polycrystalline SnSe doped with Ag,” J. Mater. Chem. A, 2, 11171 (2014).
- ↑ Tritt, Terry M.; Subramanian, M. A. (2011). "Thermoelectric Materials, Phenomena, and Applications: A Bird's Eye View" (PDF). MRS Bulletin. 31 (3): 188. doi:10.1557/mrs2006.44.
- ↑ Labudovic, M.; Li, J. (2004). "Modeling of TE cooling of pump lasers". IEEE Transactions on Components and Packaging Technologies. 27 (4): 724. doi:10.1109/TCAPT.2004.838874.
- 1 2 Yang, J. (2005). "ICT 2005. 24th International Conference on Thermoelectrics, 2005": 170. doi:10.1109/ICT.2005.1519911. ISBN 0-7803-9552-2.
|chapter=ignored (help) - ↑ Fairbanks, J., Thermoelectric Developments for Vehicular Applications, U.S. Department of Energy: Energy Efficiency and Renewable Energy. Presented on: August 24, 2006.
- ↑ Goldsmid, H.J.; Giutronich, J.E.; Kaila, M.M. (1980). "Thermoelectrics: Direct Solar Thermal Energy Conversion" (PDF). Solar Energy. 24 (5): 435. Bibcode:1980SoEn...24..435G. doi:10.1016/0038-092X(80)90311-4.
Bibliography
- Rowe, D.M. (9 December 2005). Thermoelectrics Handbook: Macro to Nano. CRC Press. ISBN 978-1-4200-3890-3.
- Taroni, Prospero J.; Hoces, Itziar; Stingelin, Natalie; Heeney, Martin; Bilotti, Emiliano (2014). "Thermoelectric Materials: A Brief Historical Survey from Metal Junctions and Inorganic Semiconductors to Organic Polymers". Israel Journal Of Chemistry. 54 (5/6). pp. 534–552. doi:10.1002/ijch.201400037.
External links
- TE Modules Application Tips and Hints
- The Seebeck Coefficient
- Materials for Thermoelectric Devices (4th chapter of Martin Wagner dissertation)