Tintinnid
| Tintinnids | |
|---|---|
 | |
| Scientific classification | |
| Domain: | Eukaryota |
| (unranked): | SAR |
| (unranked): | Alveolata |
| Phylum: | Ciliophora |
| Class: | Spirotrichea |
| Subclass: | Choreotrichia |
| Order: | Tintinnida Kofoid & Campbell, 1929 |
Tintinnids are ciliates of the choreotrich taxon Tintinnida, distinguished by vase-shaped shells, the name deriving from a Latin source meaning a small tinkling bell, that are called loricae,, which are mostly protein but may incorporate minute pieces of minerals (Agatha, Laval-Peuto & Simon 2013). Fossils resembling tintinnid loricas in shape and size, Calpionellids, appear as early as the Ordovician period but are formed of calcite and as no extant ciliate taxa forms calcite shells they are unlikely to be tintinnids and probably not ciliates at all (Remane 1985). Fossils which can be reliably related to extant tintinnids (e.g. fossils of aggultinated lorica) are in the fossil record during the Jurassic but do not become abundant until the Cretaceous (Lipps, Stoeck & Dunthorn 2013). Tintinnids are an important part of the fossil record because of the rarity with which most other ciliates become preserved under the conditions of the marine environment. The loricae of some tintinnids are easily preserved, giving them a relatively good fossil record.

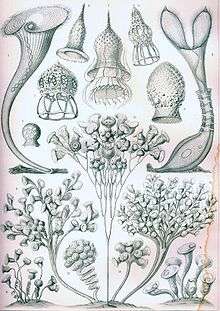
Tintinnid loricas or shells show an amazing variety of styles. They were among the many planktonic microorganisms featured in Ernst's Haeckel's classic work popularizing the beauty of the natural world "Art forms in Nature" Kunstformen der Natur.
Like other protists, tintinnids are complex single-cell (Eukaryota) organisms. Tintinnids are heterotrophic aquatic organisms. They feed primarily on photosynthetic algae and bacteria (Montagnes 2013). They are part of the microzooplankton (between 20 and 200 micrometres in size). Tintinnids are found in marine and freshwaters. However, they are most common in salt water and are usually present in concentrations of about 100 a liter but can reach abundances of several thousand per litre (McManus & Santoferrara 2013). Characteristics of their lorica, or shells, are classically used to distinguish the roughly 1000 species described. However, in recent years application of histological and molecular techniques have led to many taxonomic revisions (Agatha & Strûder-Kypke 2013).
Many species appear to have wide distributions (for example from the Chesapeake Bay to New Caledonia) while others are restricted to certain areas, such as arctic waters or coastal seas (Dolan & Pierce 2013). Nonetheless, in any given locale dozens of species can be found. Like other members of the microzooplankton (such as oligotrich ciliates, heterotrophic dinoflagellates, radiolarians, etc.), tintinnids are a vital link in aquatic food chains as they are the 'herbivores' of the plankton. They feed on phytoplankton (algae and cyanobacteria) and in turn act as food for larger organisms such as copepods (small crustaceans) and larval fish (Stoecker 2013).
The color image on the right is a specimen of Dictyocysta mitra from the Bay of Villefranche in the Mediterranean Sea. The hair-like projections pointing out of the top of the shell are the cilia of the cell. The cilia generate a water flow across the mouth of the cell, bringing food into contact and move the tintinnid. Their swimming pattern is rather 'jumpy'- or dancing- they are part of the 'choreotrichs' which means dancing hairs from their swimming behaviour and cilia (Montagnes 2013).
 Life history stages of a tintinnid, Eutintinnus inquilinus. In ciliates reproduction (B&C) is divorced from sexual recombination (D&E).
Life history stages of a tintinnid, Eutintinnus inquilinus. In ciliates reproduction (B&C) is divorced from sexual recombination (D&E). Living cells from the Bay of Villefranche (N.W. Mediterranean Sea)
Living cells from the Bay of Villefranche (N.W. Mediterranean Sea) Tintinnids of the California Current Ecosystem
Tintinnids of the California Current Ecosystem Amundsen Sea (Antarctica) Tintinnids
Amundsen Sea (Antarctica) Tintinnids Images of tintinnids and other microplankton found in the Chukchi Sea in the Arctic
Images of tintinnids and other microplankton found in the Chukchi Sea in the Arctic Tintinnids of the Thau Lagoon (Sète, France)
Tintinnids of the Thau Lagoon (Sète, France) Tintinnids from the estuarine region of the Ganges River in India
Tintinnids from the estuarine region of the Ganges River in India
References
Agatha S, Laval-Peuto M, Simon P. 2013. The tintinnid lorica. In: Dolan JR, Montagnes DJS, Agatha S, Coats WD, Stoecker DK, editors. The biology and ecology of tintinnid ciliates: models for marine plankton. West Sussex: Wiley-Blackwell. p. 17-41.
Agatha, S. Strûder-Kypke, M.C. 2013. Systematics and Evolution of tintinnid ciliates. In: Dolan JR, Montagnes DJS, Agatha S, Coats WD, Stoecker DK, editors. The biology and ecology of tintinnid ciliates: models for marine plankton. West Sussex: Wiley-Blackwell. p. 42-84.
Dolan, J.R., Pierce, R.W. 2013. Diversity and distributions of tintinnds. In: Dolan JR, Montagnes DJS, Agatha S, Coats WD, Stoecker DK, editors. The biology and ecology of tintinnid ciliates: models for marine plankton. West Sussex: Wiley-Blackwell. p. 214-243.
Fauré-Fremiet, E. 1924. Contribution à la connaissance des infusoires planktoniques. Bulletin Biologique de France et de Belgique, Suppl. 6, 1-171.
Lipps J.H., Stoeck T., Dunthorn M. 2013. Fossil tintinnids. In: Dolan J, Montagnes D, Agatha S, Coats W, Stoecker D, editors. Biology and ecology of tintinnid ciliates: models for marine plankton. West Sussex: Wiley-Blackwell. p. 186-197.
McManus, G.B., Santoferrara, L.F. 2013. Tintinnids in microzooplankton communities. In: Dolan JR, Montagnes DJS, Agatha S, Coats WD, Stoecker DK, editors. The biology and ecology of tintinnid ciliates: models for marine plankton. West Sussex: Wiley-Blackwell. p. 198-213.
Montagnes, D.J.S. 2013. Ecophysiology and behavior of tintinnids. In: Dolan JR, Montagnes DJS, Agatha S, Coats WD, Stoecker DK, editors. The biology and ecology of tintinnid ciliates: models for marine plankton. West Sussex: Wiley-Blackwell. p. 85-121.
Remane, J. 1985. Calpionellids. In: Plankton Stratigraphy, H.M. Bolli, J. B. Saunders, and K. Perch-Nielsen eds., pp 555-572. Cambridge University Press, Cambridge, U.K.
Stoecker, D.K. 2013. Predators of tintinnids. In: Dolan JR, Montagnes DJS, Agatha S, Coats WD, Stoecker DK, editors. The biology and ecology of tintinnid ciliates: models for marine plankton. West Sussex: Wiley-Blackwell. p. 122-144.