Triglycine sulfate
| Names | |
|---|---|
| IUPAC name
Glycine sulfate (3:1) | |
| Other names
Glycine sulfate; TGS | |
| Identifiers | |
| 513-29-1 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 10111 |
| ECHA InfoCard | 100.007.414 |
| PubChem | 10551 |
| |
| |
| Properties | |
| C6H17N3O10S | |
| Molar mass | 323.27 g·mol−1 |
| Appearance | White powder |
| Density | 1.69 g/cm3[1] |
| Structure | |
| Monoclinic | |
| P21[2] | |
| a = 0.9417 nm, b = 1.2643 nm, c = 0.5735 nm α = 90°, β = 110°, γ = 90° | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Triglycine sulfate (TGS) is a chemical compound with a formula (NH2CH2COOH)3·H2SO4. The empirical formula of TGS does not represent the molecular structure, which contains protonated glycine moieties and sulfate ions. TGS with protons replaced by deuterium is called deuterated TGS or DTGS; alternatively, DTGS may refer to doped TGS. TGS and DTGS crystals are pyroelectric and ferroelectric and have been used as detector elements in infrared spectroscopy.
Crystal structure and properties
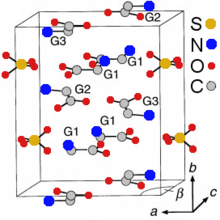
TGS crystals may be formed by evaporation of an aqueous solution of sulfuric acid, which is containing a greater than three-fold excess of glycine.[3] They belong to the polar space group P21 and therefore are pyroelectric and ferroelectric at room temperature, exhibiting spontaneous polarization along the b-axis ([010] direction). The Curie temperature of the ferroelectric transition is 49 °C for TGS and 62 °C for DTGS. The crystal structure consists of SO42−, 2(N+H3CH2COOH) (G1 and G2 in the crystal-structure diagram), and +NH3CH2COO− (G3) species held together by hydrogen bonds.[4] These bonds are easily broken by the polar molecules of water that explains the hygroscopicity of TGS – its crystals are easily etched by water. Along the b-axis, the G1-SO4 and G2-G3 layers are stacked alternately. The nearest two neighboring layers with identical chemical composition are rotated 180° around the b-axis against each other.[2][5]
References
- ↑ Kwan-Chi Kao (2004). Dielectric phenomena in solids: with emphasis on physical concepts of electronic processes. Academic Press. pp. 318–. ISBN 978-0-12-396561-5. Retrieved 12 May 2011.
- 1 2 3 Subramanian Balakumar and Hua C. Zeng (2000). "Water-assisted reconstruction on ferroelectric domain ends of triglycine sulfate (NH2CH2COOH)3·H2SO4 crystals". J. Mater. Chem. 10: 651–656. doi:10.1039/A907937H.
- ↑ Pandya, G. R.; Vyas, D.D (1980). "Crystallization of glycine-sulfate". Journal of Crystal Growth. 5 (4): 870–872. Bibcode:1980JCrGr..50..870P. doi:10.1016/0022-0248(80)90150-5.
- ↑ Choudhury, Rajul Ranjan; Chitra, R. (2008). "Single crystal neutron diffraction study of triglycine sulphate revisited". Pramana. 71 (5): 911–915. Bibcode:2009Prama..71..911C. doi:10.1007/s12043-008-0199-5.
- ↑ Wood, E.A.; Holden, A.N. (1957). "Monoclinic glycine sulfate: crystallographic data". Acta Crystallogr. 10: 145–146. doi:10.1107/S0365110X57000481.