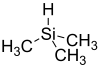
Trimethylsilane
| |||
| Identifiers | |||
|---|---|---|---|
| 993-07-7 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 63614 | ||
| ECHA InfoCard | 100.012.366 | ||
| |||
| Properties | |||
| C3H10Si | |||
| Molar mass | 74.20 g·mol−1 | ||
| Density | 0.638 g cm−3 | ||
| Melting point | −135.9 °C (−212.6 °F; 137.2 K) | ||
| Boiling point | 6.7 °C (44.1 °F; 279.8 K) | ||
| Hazards | |||
| EU classification (DSD) |
| ||
| R-phrases | R12, R36/37/38 | ||
| S-phrases | S9, S16, S26, S33 | ||
| NFPA 704 | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Trimethylsilane or trimethylsilyl hydride, is a compound with the formula C3H10Si, or with the structural formula (CH3)3SiH. It is very flammable. Trimethylsilane is used in the semi-conductor industry as an etchant in the plasma phase.[1]
See also
- Dimethylsilane
- Trimethylsilyl functional group
References
- ↑ Chen, Sheng-Wen; Wang, Yu-Sheng; Hu, Shao-Yu; Lee, Wen-Hsi; Chi, Chieh-Cheng; Wang, Ying-Lang (2012). "A Study of Trimethylsilane (3MS) and Tetramethylsilane (4MS) Based α-SiCN:H/α-SiCO:H Diffusion Barrier Films". Materials. 5 (3): 377. doi:10.3390/ma5030377.
This article is issued from Wikipedia - version of the 11/16/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.