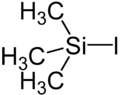
Trimethylsilyl iodide
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Iodo(trimethyl)silane | |||
| Identifiers | |||
| 16029-98-4 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 76879 | ||
| ECHA InfoCard | 100.036.503 | ||
| PubChem | 85247 | ||
| |||
| |||
| Properties | |||
| C3H9ISi | |||
| Molar mass | 200.09 g·mol−1 | ||
| Density | 1.406 g/mL at 25 °C | ||
| Boiling point | 106 °C (223 °F; 379 K) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Trimethylsilyl iodide, (CH3)3SiI, iodotrimethylsilane or TMSI, is an organosilicon compound. It is a colorless, volatile liquid at room temperature.
Preparation
Trimethylsilyl iodide may be prepared by the oxidative cleavage of hexamethyldisilane by iodine,[1] or by the cleavage of hexamethyldisiloxane with aluminium triiodide.[1][2]
- TMS-TMS + I2 → 2 TMSI (TMS = (CH3)3Si)
- TMS-O-TMS + AlI3 → 2 TMSI + AlIO
Applications
Trimethylsilyl iodide is used to introduce the trimethylsilyl group onto alcohols (ROH):
- ROH + TMSI → RO(TMS) + HI
This type of reaction may be useful for gas chromatography analysis; the resultant silyl ether is more volatile than the underivatized original materials.[3]
TMSI reacts with alkyl ethers (ROR′), forming silyl ethers (ROSiMe3) and iodoalkanes (RI) that can be hydrolysed to alcohols (ROH).[4]
For the preparation of bulk trimethylsilylated material, trimethylsilyl chloride may be preferred due to its lower cost.
Trimethylsilyl iodide is also used for the removal of the Boc protecting group,[1][5][6] especially where other deprotection methods are too harsh for the substrate.[7]
References
- 1 2 3 Olah, G; Narang, S. C. (1982). "Iodotrimethylsilane—a versatile synthetic reagent". Tetrahedron. 38 (15): 2225. doi:10.1016/0040-4020(82)87002-6.
- ↑ Michael E. Jung; Mark A. Lyster (1988). "Cleavage of Methyl Ethers with Iodotrimethylsilane: Cyclohexanol from Cyclohexyl Methyl Ether". Org. Synth.; Coll. Vol., 6, p. 353
- ↑ http://www.chem.agilent.com/Library/applications/gcms59_opiates_urine.pdf
- ↑ Michael E. Jung; Mark A. Lyster (1977). "Quantitative dealkylation of alkyl ethers via treatment with trimethylsilyl iodide. A new method for ether hydrolysis". J. Org. Chem. 42: 3761–3764. doi:10.1021/jo00443a033.
- ↑ Michael E. Jung; Mark A. Lyster (1978). "Conversion of alkyl carbamates into amines via treatment with trimethylsilyl iodide". J. Chem. Soc., Chem. Commun.: 315–316. doi:10.1039/C39780000315.
- ↑ Richard S. Lott; Virander S. Chauhan; Charles H. Stammer (1979). "Trimethylsilyl iodide as a peptide deblocking agent". J. Chem. Soc., Chem. Commun.: 495–496. doi:10.1039/C39790000495.
- ↑ Zhijian Liu; Nobuyoshi Yasuda; Michael Simeone; Robert A. Reamer (2014). "N-Boc Deprotection and Isolation Method for Water-Soluble Zwitterionic Compounds". J. Org. Chem. 79: 11792–11796. doi:10.1021/jo502319z.