Vinyl bromide
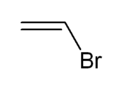 | |
| Names | |
|---|---|
| IUPAC name
Bromoethene | |
| Other names
Vinyl bromide, 1-Bromoethene, Bromoethylene, 1-Bromoethylene, Monobromoethene, Monobromoethylene, R1140 B1, UN 1085 | |
| Identifiers | |
| 593-60-2 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 11151 |
| ECHA InfoCard | 100.008.911 |
| EC Number | 209-800-6 |
| KEGG | C19184 |
| PubChem | 11641 |
| RTECS number | KU8400000 |
| |
| |
| Properties | |
| C2H3Br | |
| Molar mass | 106.95 g/mol |
| Appearance | Colorless gas |
| Odor | pleasant[1] |
| Density | 1.525 g/cm3 at boiling point (liquid)
1.4933 g/cm3 at 20 °C |
| Melting point | −137.8 °C (−216.0 °F; 135.3 K) |
| Boiling point | 15.8 °C (60.4 °F; 288.9 K) |
| Insoluble | |
| log P | 1.57 |
| Vapor pressure | 206.8 kPa at 37.8 °C |
| Hazards | |
| Main hazards | Toxic (T), Highly flammable (F+) |
| Safety data sheet | See: data page |
| R-phrases | R12, R20/21/22, R36/37/38, R45 |
| S-phrases | S45, S53 |
| NFPA 704 | |
| Flash point | 5 °C (41 °F; 278 K) |
| 530 °C (986 °F; 803 K) | |
| Explosive limits | 9%-15%[1] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
none[1] |
| REL (Recommended) |
Ca[1] |
| IDLH (Immediate danger) |
N.D.[1] |
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. | |
| Thermodynamic data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Vinyl bromide is a simple vinyl halide. It is soluble in chloroform, ethanol, diethyl ether, tetrahydrofuran, acetone and benzene.
Uses
Vinyl bromide is used to manufacture bromopolymers and mainly polyvinyl bromide. Further it is used as an alkylation agent.
Safety precautions
Vinyl bromide is highly flammable liquid and reacts violently with oxidizers.
It is listed in List of IARC Group 2A carcinogens as a suspected human carcinogen.
See also
References
External links
- International Chemical Safety Card 0597
- "NIOSH Pocket Guide to Chemical Hazards #0657". National Institute for Occupational Safety and Health (NIOSH).
- MSDS at Oxford University
- MSDS at mathesontrigas.com
- Vinyl bromide at IRIS
- Vinyl bromide at osha.gov
- IARC Summary & Evaluation of vinyl bromide
- Report on Carcinogens Background Document for Vinyl Bromide
- Synthesis of vinyl bromides
- The Kinetics of Pyrolysis of Vinyl Bromide
- UV absorption spectra
- UV Spectrum and Cross Sections
- 1H NMR spectrum
This article is issued from Wikipedia - version of the 3/16/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
