Zingerone
 | |
| Names | |
|---|---|
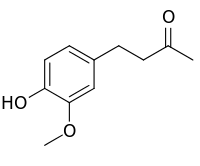
| IUPAC name
4-(4-hydroxy-3-methoxyphenyl)-2-butanone | |
| Identifiers | |
| 122-48-5 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:68657 |
| ChEMBL | ChEMBL25894 |
| ChemSpider | 28952 |
| ECHA InfoCard | 100.004.136 |
| PubChem | 31211 |
| UNII | 4MMW850892 |
| |
| |
| Properties | |
| C11H14O3 | |
| Molar mass | 194.22 g/mol |
| Melting point | 40 to 41 °C (104 to 106 °F; 313 to 314 K) |
| Boiling point | 187 to 188 °C (369 to 370 °F; 460 to 461 K) at 14 mmHg |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Zingerone, also called vanillylacetone, is a thought by some to be a key component of the pungency of ginger, but imparts the "sweet" flavor of cooked ginger.[1] Certainly Zingerone is a crystalline solid that is sparingly soluble in water, but soluble in ether, however when synthesised and tasted does not have any pungency, which suggests it is more likely that zingerone is a decomposition product of, rather than the direct source of the pungency of ginger.[2]
Zingerone is similar in chemical structure to other flavor chemicals such as vanillin and eugenol. It is used as a flavor additive in spice oils and in perfumery to introduce spicy aromas.
Fresh ginger does not contain zingerone, but it is produced by cooking which performs a reverse aldol reaction on gingerol.
Biological effects
Ginger compounds have been shown to be active against enterotoxigenic Escherichia coli heat-labile enterotoxin-induced diarrhea. This type of diarrhea is the leading cause of infant death in developing countries. Zingerone is likely the active constituent responsible for the antidiarrheal efficacy of ginger.[3]
Zingerone also stimulates the release of catecholamines, which aids in breakdown of fat cells and zingerone was shown to inhibit obesity-induced inflammation.[4]
References
- ↑ Monge, P; Scheline, R; Solheim, E (1976). "The metabolism of zingerone, a pungent principle of ginger". Xenobiotica. 6 (7): 411–23. doi:10.3109/00498257609151654. PMID 997589.
- ↑ Steffen Arctander, Perfume and Flavor Materials of Natural Origin, pg. 280
- ↑ Chen, Jaw-Chyun; Li-Jiau Huang; Shih-Lu Wu; Sheng-Chu Kuo; Tin-Yun Ho; Chien-Yun Hsiang (2007). "Ginger and Its Bioactive Component Inhibit Enterotoxigenic Escherichia coli Heat-Labile Enterotoxin-Induced Diarrhea in Mice". Journal of Agricultural and Food Chemistry. 55 (21): 8390–7. doi:10.1021/jf071460f. PMID 17880155.
- ↑ Pulbutr P. et al. Lipolytic Effects of zingerone in adipocytes isolated from normal diet-fed rats and high fat diet-fed rats. International Journal of Pharmacology. Jul 2011; 7(5):29-34