δ34S
The δ34S (pronounced delta 34 S) value is a standardized method for reporting measurements of the ratio of two stable isotopes of sulfur, 34S:32S, in a sample against the equivalent ratio in a known reference standard. Presently, the most commonly used standard is Vienna-Canyon Diablo Troilite (VCDT). Results are reported as variations from the standard ratio in parts per thousand, per mil or per mille, using the ‰ symbol. Heavy and light sulfur isotopes fractionate at different rates and the resulting δ34S values, recorded in marine sulfate or sedimentary sulfides, have been studied and interpreted as records of the changing sulfur cycle throughout the earth's history.
Calculation
Of the 25 known isotopes of sulfur, four are stable.[2] In order of their abundance, those isotopes are 32S (94.93%), 34S (4.29%), 33S (0.76%), and 36S (0.02%).[3] The δ34S value refers to a measure of the ratio of the two most common stable sulfur isotopes, 34S:32S, as measured in a sample against that same ratio as measured in a known reference standard. The lowercase delta character is used by convention, to be consistent with use in other areas of stable isotope chemistry. That value can be calculated in per mil (‰, parts per thousand) as:[4]
Less commonly, if the appropriate isotope abundances are measured, similar formulae can be used to quantify ratio variations between 33S and 32S, and 36S and 32S, reported as δ33S and δ36S, respectively.[5]
Reference standard
Sulfur from meteorites was determined in the early 1950s to be an adequate reference standard because it exhibited a small variability in isotopic ratios.[6] It was also believed that because of their extraterrestrial provenances, meteors represented primordial terrestrial isotopic conditions.[1] During a meeting of the National Science Foundation in April 1962, troilite from the Canyon Diablo meteorite found in Arizona, US, was established as the standard with which δ34S values (and other sulfur stable isotopic ratios) could be calculated.[6][7] Known as Canyon Diablo Troilite (CDT), the standard was established as having a 32S:34S ratio of 22.220 and was used for around three decades.[6] In 1993, the International Atomic Energy Agency (IAEA) established a new standard, Vienna-CDT (VCDT), based on artificially prepared silver sulfide (IAEA-S-1) that was defined to have a δ34SVCDT value of −0.3‰.[7] In 1994, the original CDT material was found not to be isotopically homogeneous, with internal variations as great as 0.4‰, confirming its unsuitability as a reference standard.[6]
Causes of variations
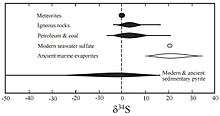
Two mechanisms of fractionation occur that alter sulfur stable isotope ratios: kinetic effects, especially due to the metabolism of sulfate-reducing bacteria, and isotope exchange reactions that occur between sulfide phases based on temperature.[8] With VCDT as the reference standard, natural δ34S value variations have been recorded between +120‰ and -65‰.[7]
The presence of sulfate-reducing bacteria, which reduce sulfate (SO2−
4) to hydrogen sulfide (H2S), has played a significant role in the oceanic δ34S value throughout the earth's history. Sulfate-reducing bacteria metabolize 32S more readily than 34S, resulting in an increase in the value of the δ34S in the remaining sulfate in the seawater.[1] Archean pyrite found in barite in the Warrawoona Group, Western Australia, with sulfur fractionations as great as 21.1‰ hint at the presence of sulfate-reducers as early as 3,470 million years ago.[9]
The δ34S value, recorded by sulfate in marine evaporites, can be used to chart the sulfur cycle throughout earth's history.[1][4] The Great Oxygenation Event around 2,400 million years ago altered the sulfur cycle radically, as increased atmospheric oxygen permitted an increase in the mechanisms that could fractionate sulfur isotopes, leading to an increase in the δ34S value from ~0‰ pre-oxygenation. Approximately 700 million years ago, the δ34S values in seawater sulfates began to vary more and those in sedimentary sulfates grew more negative. Researchers have interpreted this excursion as indicative of an increase in water column oxygenation with continued periods of anoxia in the deepest waters. Modern seawater sulfate δ34S values are consistently 21.0 ± 0.2‰ across the world's oceans, while sedimentary sulfides vary widely. Seawater sulfate δ34S and δ18O values exhibit similar trends not seen in sedimentary sulfide minerals.[1]
See also
References
- 1 2 3 4 5 Seal, II, R. R. (2006). "Sulfur Isotope Geochemistry of Sulfide Minerals". Reviews in Mineralogy and Geochemistry. 61 (1): 633–677. doi:10.2138/rmg.2006.61.12.

- ↑ Audi, G.; Bersillon, O.; Blachot, J.; Wapstra, A. H. (December 2003). "The NUBASE evaluation of nuclear and decay properties". Nuclear Physics A. 729 (1): 3–128. doi:10.1016/j.nuclphysa.2003.11.001.

- ↑ Hoefs 2009, p. 71.
- 1 2 Canfield, D. E. (2001). "Biogeochemistry of Sulfur Isotopes". Reviews in Mineralogy and Geochemistry. 43 (1): 607–636. doi:10.2138/gsrmg.43.1.607.

- ↑ Whitehouse, M. J. (March 2013). "Multiple Sulfur Isotope Determination by SIMS: Evaluation of Reference Sulfides for Δ33S with Observations and a Case Study on the Determination of Δ36S". Geostandards and Geoanalytical Research. 37 (1): 19–33. doi:10.1111/j.1751-908X.2012.00188.x.

- 1 2 3 4 Beaudoin, G.; Taylor, B. E.; Rumble, III, D.; Thiemens, M. (October 1994). "Variations in the sulfur isotope composition of troilite from the Cañon Diablo iron meteorite". Geochimica et Cosmochimica Acta. 58 (19): 4253–4255. doi:10.1016/0016-7037(94)90277-1.

- 1 2 3 Hoefs 2009, p. 72.
- ↑ Hoefs 2009, pp. 73, 77.
- ↑ Shen, Y.; Buick, R.; Canfield, D. E. (March 2001). "Isotopic evidence for microbial sulphate reduction in the early Archaean era". Nature. 410 (6824): 77–81. doi:10.1038/35065071.

Citations
- Hoefs, J. (2009). Stable Isotope Geochemistry (6th ed.). Berlin: Springer-Verlag. ISBN 978-3-540-70703-5.