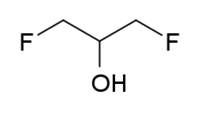
1,3-Difluoro-2-propanol
 | |
| Names | |
|---|---|
| IUPAC name
1,3-Difluoro-2-propanol | |
| Identifiers | |
| 453-13-4 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChemSpider | 61300 |
| ECHA InfoCard | 100.006.561 |
| PubChem | 67985 |
| |
| |
| Properties | |
| C3H6F2O | |
| Molar mass | 96.08 g·mol−1 |
| Density | 1.24 g/cm3 (at 25 °C) [1] |
| Boiling point | 54 to 55 °C (129 to 131 °F; 327 to 328 K) |
| Hazards | |
| Flash point | 42 °C (108 °F; 315 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
1,3-Difluoro-2-propanol is a metabolic poison which disrupts the citric acid cycle and is used as a rodenticide, similar to sodium fluoroacetate. It is the main ingredient (along with 1-chloro-3-fluoro-2-propanol) in the rodenticide product Gliftor which was widely used in the former USSR.[2][3][4]
References
- ↑ Sigma Aldrich
- ↑ Buklan AI, Kravets AF (1986). "[Gliftor poisoning]". Sud. Med. Ekspert. (in Russian). 29 (1): 55–6. PMID 3961873.
- ↑ Feldwick MG, Noakes PS, Prause U, Mead RJ, Kostyniak PJ (1998). "The biochemical toxicology of 1,3-difluoro-2-propanol, the major ingredient of the pesticide gliftor: the potential of 4-methylpyrazole as an antidote". J. Biochem. Mol. Toxicol. 12 (1): 41–52. doi:10.1002/(SICI)1099-0461(1998)12:1<41::AID-JBT6>3.0.CO;2-P. PMID 9414486.
- ↑ Menon KI, Feldwick MG, Noakes PS, Mead RJ (2001). "The mode of toxic action of the pesticide gliftor: the metabolism of 1,3-difluoroacetone to (−)-erythro-fluorocitrate". J. Biochem. Mol. Toxicol. 15 (1): 47–54. doi:10.1002/1099-0461(2001)15:1<47::AID-JBT6>3.0.CO;2-E. PMID 11170315.
This article is issued from Wikipedia - version of the 5/12/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.