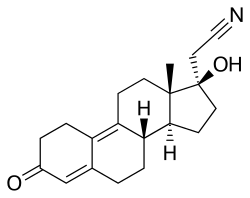
Dienogest
 | |
| Clinical data | |
|---|---|
| Trade names | Visanne; With EV: Natazia, Qlaira; With EE: Valette |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code |
G03DB08 (WHO) G03AB08 (WHO) G03FA15 (WHO) (combinations with estrogen) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 90%[1] |
| Protein binding |
Albumin (90%);[1] Free (10%)[1] |
| Metabolism | Hepatic (CYP3A4)[1][2] |
| Metabolites | Inactive[1] |
| Biological half-life | 10 hours[1] |
| Excretion | Renal |
| Identifiers | |
| |
| Synonyms | M-18575, MJR-35, SH-660, STS-557, ZK-37659; 17α-Cyanomethyl-17β-hydroxy-estra-4,9(10)-dien-3-one; 17-Hydroxy-3-oxo-19-nor-17α-pregna-4,9-diene-21-nitrile |
| CAS Number |
65928-58-7 |
| PubChem (CID) | 68861 |
| IUPHAR/BPS | 7654 |
| ChemSpider |
62093 |
| UNII |
46M3EV8HHE |
| KEGG |
D03799 |
| ChEBI |
CHEBI:70708 |
| ChEMBL |
CHEMBL1201864 |
| Chemical and physical data | |
| Formula | C20H25NO2 |
| Molar mass | 311.42 g/mol[3] |
| Density | 1.2 g/cm3 |
| Boiling point | 549 °C (1,020 °F) |
| | |
Dienogest (INN, USAN, BAN, JAN) is a steroidal progestin of the 19-nortestosterone group.[4][5] It is available in combination with estradiol valerate (as Natazia, Qlaira) or ethinyl estradiol (as Valette) for use as an oral contraceptive, and by itself (as Visanne, Dinagest) for the treatment of endometriosis in Europe, Australia, and Japan.[6] Although available in combination with estrogen as a contraceptive in the United States, dienogest is not available in this country by itself.[5] In addition to its progestogenic effects, dienogest has antiandrogenic activity, and as a result can improve androgenic symptoms such as acne.[3][7] It is a non-ethinylated progestin which is structurally related to testosterone.[2]
Medical uses
Contraception
Dienogest is used primarily as a contraceptive in combination with ethinyl estradiol. It is given as a tablet containing 2 mg of dienogest and 30 μg of ethinyl estradiol.[8] Dienogest is also available in a quadriphasic oral contraceptive pill combined with estradiol valerate, marketed as Natazia in the United States and Qlaira in some European countries and Russia. This formulation is also approved for the treatment of heavy menstrual bleeding.
Endometriosis
Dienogest is also approved in Europe, Australia, Malaysia, Singapore and Japan for the treatment of endometriosis.[6][9][10] It has been shown to be equally effective as leuprorelin,[11] which is a second line medication against endometriosis.
Side effects
Adverse effects associated with dienogest are the same as those expected of a progestogen.[3] These include weight gain, increased blood pressure, breast tenderness and nausea.[12] It produces no androgenic side effects and has little effect on metabolic and lipid haemostatic parameters.[13]
Pharmacology
Dienogest has relatively low affinity for the progesterone receptor (PR) in vitro in human uterus tissue, about 10% that of progesterone.[1][14] In spite of its relatively weak affinity for the PR, the drug shows strong progestogenic effects on the endometrium.[1][15] Dienogest is said to be one of the only 19-nortestosterone derivative progestins that does not have androgenic properties.[15] In fact, it actually has antiandrogen activity, about 30–40% of that of cyproterone acetate,[1][15] and can improve androgenic symptoms such as acne and hirsutism.[3][7] The drug does not interact with the estrogen, glucocorticoid, or mineralocorticoid receptor,[1] and hence has no estrogenic, glucocorticoid, or antimineralocorticoid activity.[15]
Metabolites of dienogest, such as 9α,10β-dihydrodienogest and 3,5α-tetrahydrodienogest, show greater affinity for the PR and AR than does dienogest itself (see table below).[16]
| Compound | PR (%) | AR (%) | |||||||
| Dienogest | 5 | 10 | |||||||
| 9α,10β-Dihydrodienogest | 26 | 13 | |||||||
| 3,5α-Tetrahydrodienogest | 19 | 16 | |||||||
| PR (promegestone = 100%), AR (metribolone = 100%) | |||||||||
Inhibition of ovulation
The minimum effective dose of oral dienogest required to inhibit ovulation is 1 mg/day.[17][18] The inhibition of ovulation by dienogest reportedly occurs mainly via peripheral action as opposed to central action on gonadotropin secretion.[3]
Oral treatment of dienogest 2 mg/day in cyclical women reduced serum progesterone levels to anovulatory levels, however serum levels of luteinizing hormone and follicle-stimulating hormone are not significantly altered.[17]
Pharmacokinetics
Dienogest is rapidly absorbed and has high bioavailability of approximately 90%.[1] It is exclusively protein-bound to albumin (90%, with the remaining 10% being free), and does not bind to sex hormone-binding globulin or corticosteroid-binding globulin.[1] The drug is metabolized in the liver mainly by CYP3A4.[1] Its metabolites are said to be inactive and to be rapidly excreted.[1] The terminal half-life of dienogest is 10 hours.[1][2] It reaches steady-state concentrations after 2 days of administration and does not accumulate in the body.[1]
Chemistry
Dienogest is an estrane (C18) steroid and 17α-substituted 19-nortestosterone (estra-4-en-17β-ol-3-one) derivative.[19] It is also known by the chemical names 17α-cyanomethyl-19-nor-δ9-testosterone and 17α-cyanomethylestra-4,9-dien-17β-ol-3-one.[3] Dienogest is the 17α-cyanomethylated derivative of the anabolic-androgenic steroid (AAS) dienolone, as well as the 17α-cyanomethyl variant of the AAS methyldienolone (17α-methyldienolone) and ethyldienolone (17α-ethyldienolone). It is unique among 19-nortestosterone progestins in that it possesses a cyanomethyl group at the C17α position (instead of the usual ethynyl group),[19] and is also unique among most 19-nortestosterone progestins in that it has a double bond between the C9 and C10 positions.[19]
Structure-activity relationships
The 17α-cyanomethyl group of dienogest is responsible for its unique antiandrogenic instead of androgenic activity.[19] A loss of ability to activate the AR is also seen with other testosterone derivatives with extended-length C17α chains such as topterone (propyltestosterone) (compare to ethyltestosterone and methyltestosterone) and allylestrenol (compare to ethylestrenol).[20][21]
Studies with steroids similar to dienogest (e.g., dienolone) have found that the introduction of a double bond between the C9 and C10 positions is associated with similar/almost unchanged affinity for the PR and AR.[22] On the other hand, the C9(10) double bond of dienogest appears to inhibit metabolism via 5α-reductase and/or 5β-reductase, which is the major metabolic route for other 19-nortestosterone progestins like norethisterone, norgestrel, and etonogestrel, and this may serve to improve the metabolic stability and potency of dienogest.[23][24]
History
Dienogest was synthesized in 1979 in Jena, Germany under the leadership of Prof. Kurt Ponsold, was initially referred to as STS-557.[25][26] It was found that its potency was 10 times that of levonorgestrel.[27] The first product on the market to contain dienogest was a combined oral contraceptive pill (with ethinyl estradiol), Valette, introduced in 1995 and made by Jenapharm.[28] In 2007, dienogest was introduced as Dinagest in Japan for the treatment of endometriosis, and it was subsequently marketed for this indication as Visanne in Europe and Australia in December 2009 and April 2010, respectively.[6] Qlaira was introduced in Europe in 2009 and Natazia was introduced in the United States in 2010.[29]
Society and culture
Availability
Dienogest is available both alone and in combination with estradiol or ethinyl estradiol throughout much of the world, including (but not limited to) Canada and many European, South American, and Southeast Asian countries.[30] Dienogest is available only in combination with an estrogen and not as a standalone drug in the United States and the United Kingdom.[30]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Bińkowska, Małgorzata; Woroń, Jarosław (2015). "Progestogens in menopausal hormone therapy". Menopausal Review. 2: 134–143. doi:10.5114/pm.2015.52154. ISSN 1643-8876.
[...] In addition to a very fast absorption rate, dienogest exhibits a very high bioavailability of around 90%. It is bound to albumins in 90%, and around 10% is in a free form. It binds neither to SHBG, nor CBG. Its metabolites are inactive and rapidly excreted. The half-life is 10 hours. Stable concentrations are achieved after two days of treatment. Dienogest does not accumulate in the body. It demonstrates a poor affinity to the PR, but has a very potent progestagenic effect in the endometrium, and causes endometrial atrophy after prolonged use. It shows antagonist activity by binding to the AR, and hence produces an antiandrogenic action equivalent to ca. 40% of the effect induced by cyproterone acetate. Dienogest does not interact with the GR, MR or ER. [...] In vivo, it has a powerful progestagenic and moderate antigonadotropic activity, without any androgenic, glucocorticoid or mineralocorticoid effects. A dose of 2 mg inhibits the growth of ovarian follicles at 10 mm and maintains the concentration of progesterone at a low level, but has a weak inhibitory effect on FSH and LH. Consequently, dienogest is believed to have a weak central antigonadotropic action, but a potent direct peripheral ovulation-inhibiting effect [8]. [...] Dienogest is metabolized chiefly via the CYP3A4 isoenzyme. [...]
- 1 2 3 Stanczyk FZ (2003). "All progestins are not created equal". Steroids. 68 (10–13): 879–90. doi:10.1016/j.steroids.2003.08.003. PMID 14667980.
- 1 2 3 4 5 6 Foster RH, Wilde MI (1998). "Dienogest". Drugs. 56 (5): 825–33; discussion 834–5. doi:10.2165/00003495-199856050-00007. PMID 9829156.
- ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 390–. ISBN 978-1-4757-2085-3.
- 1 2 Tommaso Falcone; William W. Hurd (22 May 2013). Clinical Reproductive Medicine and Surgery: A Practical Guide. Springer Science & Business Media. pp. 300–. ISBN 978-1-4614-6837-0.
Dienogest is a 19-nortestosterone derivative that is approved in the European Union for the treatment of endometriosis. It is not available in the United States as a separate drug. It is only available in the oral contraceptive Natazia (Bayer Pharmaceuticals, Montville, NJ, USA) (estradiol valerate/dienogest), which is a newer four-phasic pack that contains dienogest.
- 1 2 3 McCormack PL (2010). "Dienogest: a review of its use in the treatment of endometriosis". Drugs. 70 (16): 2073–88. doi:10.2165/11206320-000000000-00000. PMID 20964453.
- 1 2 Raudrant D, Rabe T (2003). "Progestogens with antiandrogenic properties". Drugs. 63 (5): 463–92. doi:10.2165/00003495-200363050-00003. PMID 12600226.
- ↑ Wiegratz I, Mittmann K, Dietrich H, Zimmermann T, Kuhl H (2006). "Fertility after discontinuation of treatment with an oral contraceptive containing 30 microg of ethinyl estradiol and 2 mg of dienogest". Fertil. Steril. 85 (6): 1812–9. doi:10.1016/j.fertnstert.2005.11.052. PMID 16759929.
- ↑ "Dienogest for the treatment of endometriosis" (PDF). London New Drugs Group. Retrieved 2010-12-07.
- ↑ "Visanne" (in German). Netdoctor.de.
- ↑ Strowitzki, T; Marr, J; Gerlinger, C; Faustmann, T; Seitz, C (2010). "Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: a 24-week, randomized, multicentre, open-label trial". Human reproduction (Oxford, England). 25 (3): 633–41. doi:10.1093/humrep/dep469. PMID 20089522.
- ↑ Galbraith, Alan; Shane Bullock; Elizabeth Manias; Barry Hunt; Ann Richards (2007). Fundamentals of Pharmacology: An Applied Approach for Nursing and Health. United Kingdom: Pearson Education LTD. p. 632. ISBN 978-0-13-186901-1.
- ↑ Wiegratz I, Lee JH, Kutschera E, Bauer HH, von Hayn C, Moore C, Mellinger U, Winkler UH, Gross W, Kuhl H (2002). "Effect of dienogest-containing oral contraceptives on lipid metabolism". Contraception. 65 (3): 223–9. doi:10.1016/S0010-7824(01)00310-9. PMID 11929644.
- ↑ Oettel M, Bervoas-Martin S, Elger W, Golbs S, Hobe G, Kaufmann G, Mathieu M, Moore C, Schneider B, Puri C, Ritter P, Reddersen G, Schon R, Strauch G, Zimmermann H (1995). "A 19-norprogestin without 17α-ethinyl group II: Dienogest from a pharmacokinetic point of view". Drugs of Today. 31 (7): 499–516.
- 1 2 3 4 Kuhl, H (2009). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric. 8 (sup1): 3–63. doi:10.1080/13697130500148875. ISSN 1369-7137.
- 1 2 Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- 1 2 Oettel M, Carol W, Elger W, Kaufmann G, Moore C, Romer W, Klinger G, Schneider B, Schroder J, Sobek L, Walter F, Zimmermann H (1995). "A 19-norprogestin without 17α-ethinyl group II: Dienogest from a pharmacodynamic point of view". Drugs of Today. 31 (7): 517–536.
- ↑ Moore C, Carol W, Gräser T, Mellinger U, Walter F (1999). "Influence of Dienogest on Ovulation in Young Fertile Women". Clinical Drug Investigation. 18 (4): 271–278. doi:10.2165/00044011-199918040-00003.
- 1 2 3 4 Bińkowska M, Woroń J (2015). "Progestogens in menopausal hormone therapy". Prz Menopauzalny. 14 (2): 134–43. doi:10.5114/pm.2015.52154. PMC 4498031
 . PMID 26327902.
. PMID 26327902. - ↑ Singh SM, Gauthier S, Labrie F (2000). "Androgen receptor antagonists (antiandrogens): structure-activity relationships". Curr. Med. Chem. 7 (2): 211–47. PMID 10637363.
- ↑ Bergink EW, Loonen PB, Kloosterboer HJ (1985). "Receptor binding of allylestrenol, a progestagen of the 19-nortestosterone series without androgenic properties". J. Steroid Biochem. 23 (2): 165–8. PMID 3928974.
- ↑ Ojasoo T, Delettré J, Mornon JP, Turpin-VanDycke C, Raynaud JP (1987). "Towards the mapping of the progesterone and androgen receptors". J. Steroid Biochem. 27 (1-3): 255–69. PMID 3695484.
- ↑ Schubert K, Schumann G, Kaufmann G (1983). "Influence of a 9-double bond on stereospecific microbial 4,5-reductions". J. Steroid Biochem. 18 (1): 75–80. PMID 6683343.
- ↑ Hobe G, Schön R, Hajek M, Undisz K, Härtl A (1998). "Studies on the hydrogenation of the progestagen dienogest in vivo and in vitro in the female rabbit". Steroids. 63 (7-8): 393–400. PMID 9654645.
- ↑ Menzenbach B; Hübner M; K. Ponsold (1984). "Untersuchungen zur Bromierung/Dehydrobromierung von 17-Cyanmethyl-17-hydroxy-östr-5(10)-en-3-on". Journal für Praktische Chemie. 326 (6): 893–898. doi:10.1002/prac.19843260606.
- ↑ Kaufmann G, Dautzenberg H, Henkel H, et al. (August 1999). "Nitrile hydratase from Rhodococcus erythropolis: metabolization of steroidal compounds with a nitrile group". Steroids. 64 (8): 535–40. doi:10.1016/S0039-128X(99)00028-8. PMID 10493599.
- ↑ Oettel M, Kurischko A (1980). "STS 557, a new orally active progestin with antiprogestational and contragestational properties in rabbits". Contraception. 21 (1): 61–9. doi:10.1016/0010-7824(80)90140-7. PMID 7357870.
- ↑ http://www.jenapharm.de/unternehmen/ueber-uns/geschichte/1965-1995/
- ↑ Guida M, Bifulco G, Di Spiezio Sardo A, Scala M, Fernandez LM, Nappi C (2010). "Review of the safety, efficacy and patient acceptability of the combined dienogest/estradiol valerate contraceptive pill". International Journal of Women's Health. 2: 279–90. doi:10.2147/IJWH.S6954. PMC 2990895
 . PMID 21151673.
. PMID 21151673. - 1 2 https://www.drugs.com/international/dienogest.html