Muricholic acid
Muricholic acids are a group of bile acids found as one of the main forms in mice, which gives them their name, and at low concentrations in other species.[1] Muricholic acids differ from the more common bile acids, such as cholic acid or chenodeoxycholic acid, by having a hydroxyl group at the 6-position. They are detectable at low concentrations in human urine.[2]
The three major bile acids in germ-free mice are cholic acid, α-muricholic, and β-muricholic acids.[3] In conventional mice, ω-muricholic acid, and various sulfated forms are also found. Conjugation with taurine (to give tauro-murocholic acids) or glycine (to give gly-murocholic acids) takes place in the liver before secretion.
Tauro-muricholic acids were shown to be potent antagonists of the bile acid receptor farnesoid X receptor (FXR).[4]
Chemical structures
-
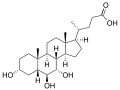
α-muricholic acid
-
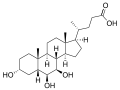
β-muricholic acid
-
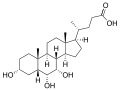
γ-muricholic acid (hyocholic acid)
-
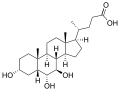
ω-muricholic acid
References
- ↑ Russell DW (2003). "The enzymes, regulation, and genetics of bile acid synthesis". Annu. Rev. Biochem. 72: 137–74. doi:10.1146/annurev.biochem.72.121801.161712. PMID 12543708.
- ↑ Goto, J; Hasegawa, K; Nambara, T; Iida, T (1992). "Studies on steroids. CCLIV. Gas chromatographic-mass spectrometric determination of 4- and 6-hydroxylated bile acids in human urine with negative ion chemical ionization detection". Journal of Chromatography. 574 (1): 1–7. PMID 1629271.
- ↑ Eyssen HJ, Parmentier GG, Mertens JA (July 1976). "Sulfate bile acids in germ-free and conventional mice". Eur. J. Biochem. 66 (3): 507–14. doi:10.1111/j.1432-1033.1976.tb10576.x. PMID 954753.
- ↑ Sayin SI, Wahlström A, Felin J, et al. (February 2013). "Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist". Cell Metab. 17 (2): 225–35. doi:10.1016/j.cmet.2013.01.003. PMID 23395169.