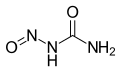
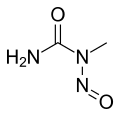
Nitrosourea
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Nitrosourea | |||
| Identifiers | |||
| 13010-20-3 | |||
| 3D model (Jmol) | Interactive image Interactive image | ||
| ChemSpider | 94772 | ||
| PubChem | 105035 | ||
| |||
| |||
| Properties | |||
| CH3N3O2 | |||
| Molar mass | 89.05 g·mol−1 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Nitrosourea is both the name of a molecule, and a class of compounds that include a nitroso (R-NO) group and a urea.
Examples
Examples include:
- Arabinopyranosyl-N-methyl-N-nitrosourea (Aranose)
- Carmustine (BCNU, BiCNU)
- Chlorozotocin
- Ethylnitrosourea (ENU)
- Fotemustine
- Lomustine (CCNU)
- Nimustine
- N-Nitroso-N-methylurea (NMU)
- Ranimustine (MCNU)
- Semustine
- Streptozocin (Streptozotocin)
Nitrosourea compounds are DNA alkylating agents and are often used in chemotherapy.[1] They are lipophilic and thus can cross the blood–brain barrier, making them useful in the treatment of brain tumors such as glioblastoma multiforme.[2]
Side effects
Some nitrosoureas (e.g. lomustine) have been associated with the development of interstitial lung disease.[3]
References
- ↑ "Antineop". Retrieved 2009-01-24.
- ↑ Takimoto CH, Calvo E. "Principles of oncologic pharmacotherapy". in Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (Eds) Cancer management: a multidisciplinary approach. 11 ed. 2008.
- ↑ Tucci E, Verdiani P, Di Carlo S, Sforza V (1986). "Lomustine (CCNU)-induced pulmonary fibrosis". Tumori. 72 (1): 95–8. PMID 3952821.
External links
- Nitrosourea Compounds at the US National Library of Medicine Medical Subject Headings (MeSH)
- DDB 9052
This article is issued from Wikipedia - version of the 8/31/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.

.svg.png)
.svg.png)

.svg.png)
.svg.png)