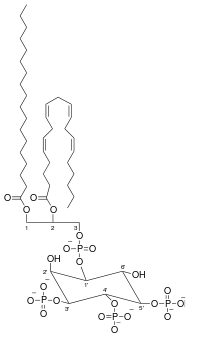
Phosphatidylinositol (3,4,5)-trisphosphate
 | |
| Names | |
|---|---|
| Other names
PI(3,4,5)P3, PtdIns(3,4,5)P3 | |
| Properties | |
| C47H86O22P4 | |
| Molar mass | 1126.46 g/mol, neutral with fatty acid composition - 18:0, 20:4 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3), abbreviated PIP3, is the product of the class I phosphoinositide 3-kinases (PI 3-kinases) phosphorylation of phosphatidylinositol (4,5)-bisphosphate (PIP2). It is a phospholipid that resides on the plasma membrane.
Discovery
In 1988, Lewis C. Cantley published a paper describing the discovery of a novel type of phosphoinositide kinase with the unprecedented ability to phosphorylate the 3' position of the inositol ring resulting in the formation of phosphatidylinositol-3-phosphate (PI3P).[1] Working independently, Alexis Traynor-Kaplan and coworkers published a paper demonstrating that a novel lipid, phosphatidylinositol 3,4,5 trisphosphate (PIP3) occurs naturally in human neutrophils with levels that increased rapidly following physiologic stimulation with chemotactic peptide.[2] Subsequent studies demonstrated that in vivo the enzyme originally identified by Cantley's group prefers PtdIns(4,5)P2 as a substrate, producing the product PIP3.[3]
Function
PIP3 functions to activate downstream signaling components, the most notable one being the protein kinase AKT, which activates downstream anabolic signaling pathways required for cell growth and survival. Phospholipase C cleaves PIP2 to produce inositol triphosphate IP3, and diacylglycerol.
PtdIns(3,4,5)P3 is dephosphorylated by the phosphatase PTEN on the 3 position, generating PI(4,5)P2, and by SHIPs (SH2-containing inositol phosphatase) on the 5' position of the inositol ring, producing PI(3,4)P2.
The PH domain in a number of proteins binds to PtdIns(3,4,5)P3. Such proteins include Akt/PKB, PDK1, Btk1, and ARNO. The generation of PtdIns(3,4,5)P3 at the plasma membrane upon the activation of class I PI 3-kinases causes these proteins to translocate to the plasma membrane and affects their activity accordingly.
The PH domain allows binding between PtdIns(3,4,5)P3 and G protein-coupled receptor kinases (GRKs). This enhances the binding of the GRK to the plasma membrane.
References
- ↑ Whitman M, Downes CP, Keeler M, Keller T, Cantley L (April 1988). "Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate". Nature. 332 (6165): 644–6. doi:10.1038/332644a0. PMID 2833705.
- ↑ Traynor-Kaplan AE, Harris AL, Thompson BL, Taylor P, Sklar LA (July 1988). "An inositol tetrakisphosphate-containing phospholipid in activated neutrophils". Nature. 334 (6180): 353–356. doi:10.1038/334353a0. PMID 3393226.
- ↑ Auger KR, Serunian LA, Soltoff SP, Libby P, Cantley LC (April 1989). "PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells". Cell. 57 (1): 167–75. doi:10.1016/0092-8674(89)90182-7. PMID 2467744.