TAR syndrome
| TAR Syndrome (Thrombocytopenia with Absent Radius) | |
|---|---|
| Classification and external resources | |
| Specialty | medical genetics |
| ICD-10 | Q87.2 |
| ICD-9-CM | 287.33 |
| OMIM | 274000 |
| DiseasesDB | 29769 |
TAR Syndrome (thrombocytopenia with absent radius) is a rare genetic disorder that is characterized by the absence of the radius bone in the forearm, and a dramatically reduced platelet count. This syndrome may occur as a part of the 1q21.1 deletion syndrome
Presentation
Symptoms of thrombocytopenia, or a lowered platelet count, leads to bruising and potentially life-threatening hemorrhage.
Other common links between people with TAR seem to include heart problems, kidney problems, knee joint problems, frequently lactose intolerance and often thumb hypoplasia
Treatment
Treatments range from platelet transfusions to surgery aimed at either centralizing the hand over the ulna to improve functionality of the hand or aimed at 'normalizing' the appearance of the arm, which is much shorter and 'clubbed.' There is some controversy surrounding the role of surgery. The infant mortality rate has been curbed by new technology, including platelet transfusions, which can even be performed in utero. The critical period is the first and sometimes second year of life. For most people with TAR, platelet counts improve as they grow out of childhood.
Genetics
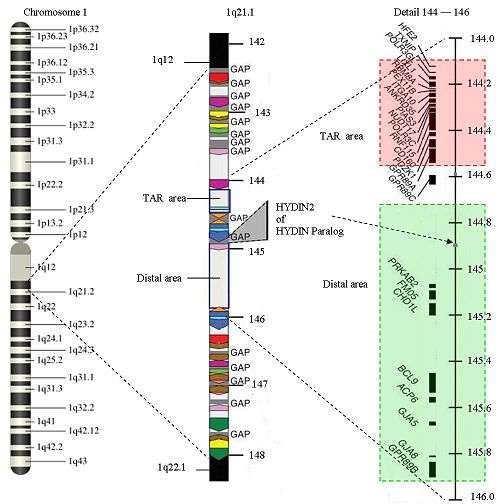
A 2007 research article identified a region of chromosome 1, 1q21.1, containing 11 genes (including HFE2, LIX1L, PIAS3, ANKRD35, ITGA10, RBM8A, PEX11B, POLR3GL, TXNIP, and GNRR2), that is heterozygously deleted in thirty of thirty patients with TAR.[1] This deletion was also found in 32% of unaffected family members, indicating that the condition requires an additional modifier. In 2012, it was discovered that TAR syndrome is a recessive condition that is caused by the person with TAR syndrome having one allele with an abnormality in the RBM8A gene that reduces, but does not eliminate entirely, the production of the protein Y14 and the other allele with the RBM8A gene either absent (due to the microdeletion identified in 2009) or, less commonly, inoperative due to a different abnormality.[2] The combination of these alleles reduces Y14 levels to a level that appears to result in the abnormalities characteristic of TAR syndrome.[3]
A study published in 2012 identified two separate RBM8A SNP abnormalities resulting in reduced Y14 production that were responsible for all but two of the cases studied, one a 5'UTR SNP with a frequency of 3.05% and the other an intronic SNP with a frequency of 0.42% in 7504 healthy individuals of the Cambridge BioResource. The microdeletion was not found in 5919 controls of the Wellcome Trust Case Control Consortium.[4]
If all TAR cases were inherited, the probability of a sibling of a person with TAR syndrome also having TAR syndrome would be 25%. However, because the RBM8A abnormalities causing TAR syndrome sometimes occur due to a mutation occurring for the first time in the person with TAR syndrome, the actual probability is somewhat lower.[5]
If both parents are carriers, at conception each child has a 25% chance of having TAR syndrome, a 50% chance of being an asymptomatic carrier, and a 25% chance of neither having TAR syndrome nor being a carrier.[6]
The children of a person with TAR syndrome will be carriers of TAR. If a person with TAR syndrome has children with a person who has a RBM8A mutation that causes reduced Y14 production, their offspring have a 50% chance of having TAR syndrome and otherwise will be carriers.[7]
Epidemiology
The incidence is 0.42 per 100,000 live births.
History
TAR was first identified in 1956, and was named almost thirteen years later when severe bruising (along with abnormally short forearms) was present in three families with nine newborns.
References
- ↑ Klopocki E, Schulze H, Strauss G, et al. (2007). "Complex Inheritance Pattern Resembling Autosomal Recessive Inheritance Involving a Microdeletion in Thrombocytopenia–Absent Radius Syndrome". Am. J. Hum. Genet. 80 (2): 232–40. doi:10.1086/510919. PMC 1785342
 . PMID 17236129.
. PMID 17236129. - ↑ Toriello HV. Thrombocytopenia Absent Radius Syndrome. 2009 Dec 8 [Updated 2012 Jun 28]. In: Pagon RA, Adam MP, Bird TD, et al., editors. GeneReviews™ [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2013. Available from: http://www.ncbi.nlm.nih.gov/books/NBK23758/
- ↑ Albers CA, Paul DS, Schulze H, Freson K, Stephens JC, Smethurst PA, Jolley JD, Cvejic A, Kostadima M, Bertone P, Breuning MH, Debili N, Deloukas P, Favier R, Fiedler J, Hobbs CM, Huang N, Hurles ME, Kiddle G, Krapels I, Nurden P, Ruivenkamp CA, Sambrook JG, Smith K, Stemple DL, Strauss G, Thys C, van Geet C, Newbury-Ecob R, Ouwehand WH, Ghevaert C (2012). "Compound inheritance of a low-frequency regulatory SNP and a rare null mutation in exon-junction complex subunit RBM8A causes TAR syndrome". Nat. Genet. 44: 435–9, S1–2. doi:10.1038/ng.1083. PMC 3428915
 . PMID 22366785.
. PMID 22366785. - ↑ Id.
- ↑ Toriello HV. Thrombocytopenia Absent Radius Syndrome. 2009 Dec 8 [Updated 2012 Jun 28]. In: Pagon RA, Adam MP, Bird TD, et al., editors. GeneReviews™ [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2013. Available from: http://www.ncbi.nlm.nih.gov/books/NBK23758/
- ↑ Id.
- ↑ Id.
Further reading
- Goldfarb, Charles A.; Wall, Lindley; Manske, Paul R. (Sep 2006). "Radial Longitudinal Deficiency: The Incidence of Associated Medical and Musculoskeletal Conditions". The Journal of Hand Surgery. 31 (7): 1176–1182. doi:10.1016/j.jhsa.2006.05.012. PMID 16945723.
External links
- Thrombocytopenia Absent Radii research study of Inherited Bone Marrow Failure Syndromes (IBMFS)
- GeneReview/NCBI/NIH/UW entry on Thrombocytopenia Absent Radius Syndrome
- These are the opinions of a young American woman who has TAR syndrome, ulnas and thumbs present (as opposed to absent). She is a 30 something mother of 3 daughters, as well as an artist and photographer in her spare time.