ThioTEPA
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a682821 |
| Pregnancy category | |
| Routes of administration | IV, intracavitary, intravesical |
| ATC code | L01AC01 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Hepatic (CYP2B, CYP3A) |
| Biological half-life | 1.5-4.1 hours |
| Excretion |
Renal 6 hours for ThioTEPA 8 hours for TEPA |
| Identifiers | |
| |
| CAS Number |
52-24-4 |
| PubChem (CID) | 5453 |
| IUPHAR/BPS | 7622 |
| DrugBank |
DB04572 |
| ChemSpider |
5254 |
| UNII |
905Z5W3GKH |
| KEGG |
D00583 |
| ChEMBL |
CHEMBL671 |
| ECHA InfoCard | 100.000.124 |
| Chemical and physical data | |
| Formula | C6H12N3PS |
| Molar mass | 189.23 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
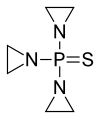
ThioTEPA or thiotepa (INN,[1] chemical name: N,N′,N″-triethylenethiophosphoramide) is an alkylating agent used to treat cancer.
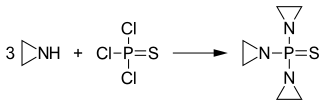
ThioTEPA is an organophosphorus compound with the formula SP(NC2H4)3.[2] It is an analogue of N,N′,N″-triethylenephosphoramide (TEPA). This molecule features tetrahedral phosphorus and is structurally akin to phosphate. It is derived from aziridine and thiophosphoryl chloride.
History and use
ThioTEPA was first developed by American Cyanamid company in the early 1950s and was reported in 1953.[3] ThioTEPA has been in use since the 1960s.
ThioTEPA has been designated as orphan drug by the European Medicines Agency on January 29, 2007, and by the United States Food and Drug Administration (FDA) on April 2, 2007, as a conditioning treatment prior to haematopoietic stem cell transplantation.[4] The applicant for these orphan drug designations was the Italian company Adienne Pharma & Biotech, owner of the drug Tepadina (thiotepa).
Thiotepa is indicated, in combination with other chemotherapy medicinal products:
- with or without total body irradiation (TBI), as conditioning treatment prior to allogeneic or autologous haematopoietic progenitor cell transplantation (HPCT) in haematological diseases in adult and paediatric patients;
- when high dose chemotherapy with HPCT support is appropriate for the treatment of solid tumours in adult and paediatric patients.[5]
Thiotepa has been previously used in the palliation of a wide variety of neoplastic diseases. The more consistent results have been seen in: adenocarcinoma of the breast, adenocarcinoma of the ovary, superficial papillary carcinoma of the urinary bladder and for controlling intracavitary effusions secondary to diffuse or localized neoplastic diseases of various serosal cavities.[5]
Thiotepa is also used as intravesical chemotherapy in bladder cancer. Three patterns of usage is identified:
- Prophylactic: before taking cystoscopic biopsy, to prevent seeding of tumor cells;
- Adjunctive: at the time of biopsy;
- Therapeutic: to prevent recurrence after cystoscopic resection of bladder tumor (transurethral resection of bladder tumor—TURBT)
For therapeutic usage, thiotepa is given in 30 mg doses intravesically weekly for 4–6 weeks. Many studies have reported up to 55% of success rate. Main toxicity of intravesical therapy is due to systemic absorption causing myelosupression, which results in thrombocytopenia and leukopenia.
Thiotepa main toxicity is myelosuppression (bone marrow depression), which is the most serious complication of excessive therapy, causing leukopenia, thrombocytopenia, and anemia.[6] Serious toxicity involving the hematologic, hepatic and respiratory system were considered as expected consequences of the conditioning regimen and transplant process.
Synthesis
References
- ↑ "International Non-Proprietary Names for Pharmaceutical Preparations. Recommended International Non-Proprietary Names (Rec. I.N.N.): List 4" (PDF). World Health Organization. March 1962. p. 111. Retrieved 27 November 2016.
- ↑ Maanen, M. J.; Smeets, C. J.; Beijnen, J. H. (2000). "Chemistry, pharmacology and pharmacokinetics of N,N',N" -triethylenethiophosphoramide (ThioTEPA)". Cancer Treatment Reviews. 26 (4): 257–268. doi:10.1053/ctrv.2000.0170. PMID 10913381.
- ↑ Sykes, M. P.; Karnofsky, D. A.; Philips, F. S.; Burchenal, J. H. (1953). "Clinical studies on triethylenephosphoramide and diethylenephosphoramide, compounds with nitrogen-mustard-like activity". Cancer. 6 (1): 142–148. doi:10.1002/1097-0142(195301)6:1<142::AID-CNCR2820060114>3.0.CO;2-W.
- ↑ "EMA Grants Adienne Marketing Rights for Tepadina". dddmag.com. Drug Discovery & Development. 19 March 2010. Retrieved 25 November 2011.
- 1 2 "URGENT – THIOTEPA UPDATE" (PDF). Food and Drug Administration. ADIENNE Pharma & Biotech. 5 April 2011. Retrieved 25 November 2011.
- ↑ Agnelli, G.; de Cunto, M.; Gresele, P.; del Favero, A. (1982). "Early onset life-threatening myelosuppression after low dose of intravesical thiotepa" (pdf). Postgraduate Medical Journal. 58 (680): 380–381. doi:10.1136/pgmj.58.680.380. PMC 2426344
 . PMID 6812036.
. PMID 6812036.
External links
- Thiotepa Official FDA information, side effects and uses on Drugs.com
- Thiotepa on cancer.org