Zinc chloride
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Zinc chloride | |
| Other names
Zinc(II) chloride Zinc dichloride Butter of zinc | |
| Identifiers | |
| 7646-85-7 Anhydrous 29426-92-4 Tetrahydrate | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:49976 |
| ChEMBL | ChEMBL1200679 |
| ChemSpider | 5525 |
| ECHA InfoCard | 100.028.720 |
| EC Number | 231-592-0 |
| PubChem | 3007855 |
| RTECS number | ZH1400000 |
| UNII | 86Q357L16B |
| UN number | 2331 |
| |
| |
| Properties | |
| ZnCl2 | |
| Molar mass | 136.315 g/mol |
| Appearance | white crystalline solid hygroscopic and very deliquescent |
| Odor | odorless |
| Density | 2.907 g/cm3 |
| Melting point | 290 °C (554 °F; 563 K)[1] |
| Boiling point | 732 °C (1,350 °F; 1,005 K)[1] |
| 432.0 g/ 1000 g (25 °C) | |
| Solubility | soluble in ethanol, glycerol and acetone |
| Solubility in alcohol | 430.0 g/100ml |
| Structure | |
| Tetrahedral, linear in the gas phase | |
| Pharmacology | |
| B05XA12 (WHO) | |
| Hazards | |
| Safety data sheet | External MSDS |
| EU classification (DSD) |
Harmful (Xn) Corrosive (C) Dangerous for the environment (N) |
| R-phrases | R22, R34, R50/53 |
| S-phrases | (S1/2), S26, S36/37/39, S45, S60, S61 |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
350 mg/kg (rat, oral) 350 mg/kg (mouse, oral) 200 mg/kg (guinea pig, oral) 1100 mg/kg (rat, oral) 1250 mg/kg (mouse, oral)[2] |
| LC50 (median concentration) |
1260 mg/m3 (rat, 30 min) 1180 mg-min/m3[2] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 1 mg/m3 (fume)[3] |
| REL (Recommended) |
TWA 1 mg/m3 ST 2 mg/m3 (fume)[3] |
| IDLH (Immediate danger) |
50 mg/m3 (fume)[3] |
| Related compounds | |
| Other anions |
Zinc fluoride Zinc bromide Zinc iodide |
| Other cations |
Cadmium chloride Mercury(II) chloride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Zinc chloride is the name of chemical compounds with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. ZnCl2 itself is hygroscopic and even deliquescent. Samples should therefore be protected from sources of moisture, including the water vapor present in ambient air. Zinc chloride finds wide application in textile processing, metallurgical fluxes, and chemical synthesis. No mineral with this chemical composition is known aside from the very rare mineral simonkolleite, Zn5(OH)8Cl2·H2O.
Structure and properties
Four crystalline forms (polymorphs) of ZnCl2 are known: α, β, γ, and δ, and in each case the Zn2+ ions are tetrahedrally coordinated to four chloride ions.[4]
| Form | Symmetry | Pearson symbol | Group | No | a (nm) | b (nm) | c (nm) | Z | ρ (g/cm3) |
|---|---|---|---|---|---|---|---|---|---|
| α | Tetragonal | tI12 | I42d | 122 | 0.5398 | 0.5398 | 0.64223 | 4 | 3.00 |
| β | Tetragonal | tP6 | P42/nmc | 137 | 0.3696 | 0.3696 | 1.071 | 2 | 3.09 |
| γ | Monoclinic | mP36 | P21/c | 14 | 0.654 | 1.131 | 1.23328 | 12 | 2.98 |
| δ | Orthorhombic | oP12 | Pna21 | 33 | 0.6125 | 0.6443 | 0.7693 | 4 | 2.98 |
Here, a, b, and c are lattice constants, Z is the number of structure units per unit cell and ρ is the density calculated from the structure parameters.[5][6][7]
The pure anhydrous orthorhombic form (δ) rapidly changes to one of the other forms on exposure to the atmosphere and a possible explanation is that the OH− ions originating from the absorbed water facilitate the rearrangement.[4] Rapid cooling of molten ZnCl2 gives a glass.[8]
The covalent character of the anhydrous material is indicated by its relatively low melting point of 275 °C.[9] Further evidence for covalency is provided by the high solubility of the dichloride in ethereal solvents where it forms adducts with the formula ZnCl2L2, where L = ligand such as O(C2H5)2. In the gas phase, ZnCl2 molecules are linear with a bond length of 205 pm.[10]
Molten ZnCl2 has a high viscosity at its melting point and a comparatively low electrical conductivity that increases markedly with temperature.[10][11] A Raman scattering study of the melt indicated the presence of polymeric structures [12] and a neutron scattering study indicated the presence of tetrahedral {ZnCl4} complexes.[13]
Hydrates
Five hydrates of zinc chloride are known, ZnCl2(H2O)n where n = 1, 1.5, 2.5, 3 and 4.[14] The tetrahydrate ZnCl2(H2O)4 crystallizes from aqueous solutions of zinc chloride.[14]
Preparation and purification
Anhydrous ZnCl2 can be prepared from zinc and hydrogen chloride.
- Zn(s) + 2 HCl → ZnCl2 + H2(g)
Hydrated forms and aqueous solutions may be readily prepared similarly by treating Zn metal with hydrochloric acid. Zinc oxide and zinc sulfide react with HCl:
Unlike many other elements, zinc essentially exists in only one oxidation state, 2+, which simplifies purification of the chloride.
Commercial samples of zinc chloride typically contain water and products from hydrolysis as impurities. Such samples may be purified by recrystallization from hot dioxane . Anhydrous samples can be purified by sublimation in a stream of hydrogen chloride gas, followed by heating the sublimate to 400 °C in a stream of dry nitrogen gas. Finally, the simplest method relies on treating the zinc chloride with thionyl chloride.[15]
Reactions
Molten anhydrous ZnCl2 at 500–700 °C dissolves zinc metal, and, on rapid cooling of the melt, a yellow diamagnetic glass is formed, which Raman studies indicate contains the Zn2+
2 ion.[14]
A number of salts containing the tetrachlorozincate anion, ZnCl2−
4, are known.[10] "Caulton's reagent," V2Cl3(thf)6Zn2Cl6 is an example of a salt containing
Zn2Cl2−
6.[16][17]
The compound Cs3ZnCl5 contains tetrahedral ZnCl2−
4 and Cl− anions.[4] No compounds containing the ZnCl4−
6 ion have been characterized.[4]
Whilst zinc chloride is very soluble in water, solutions cannot be considered to contain simply solvated Zn2+ ions and Cl− ions, ZnClxH2O(4−x) species are also present.[18][19][20] Aqueous solutions of ZnCl2 are acidic: a 6 M aqueous solution has a pH of 1.[14] The acidity of aqueous ZnCl2 solutions relative to solutions of other Zn2+ salts is due to the formation of the tetrahedral chloro aqua complexes where the reduction in coordination number from 6 to 4 further reduces the strength of the O-H bonds in the solvated water molecules.[21]
In alkali solution in the presence of OH− ion various zinc hydroxychloride anions are present in solution, e.g.Zn(OH)3Cl2−, Zn(OH)2Cl2−
2, ZnOHCl2−
3, and Zn5(OH)8Cl2·H2O (simonkolleite) precipitates.[22]
When ammonia is bubbled through a solution of zinc chloride the hydroxide does not precipitate, instead compounds containing complexed ammonia (ammines) are produced, Zn(NH3)4Cl2•H2O and on concentration ZnCl2(NH3)2.[23] The former contains the Zn(NH3)62+ ion [4] and the latter is molecular with a distorted tetrahedral geometry.[24] The species in aqueous solution have been investigated and show that Zn(NH3)42+ is the main species present with Zn(NH3)3Cl+ also present at lower NH3:Zn ratio.[25]
Aqueous zinc chloride reacts with zinc oxide to form an amorphous cement that was first investigated in the 1855 by Stanislas Sorel. Sorel later went on to investigate the related magnesium oxychloride cement, which bears his name.[26]
When hydrated zinc chloride is heated, one obtains a residue of Zn(OH)Cl e.g.[27]
- ZnCl2•2H2O → ZnCl(OH) + HCl + H2O
The compound ZnCl2·½HCl·H2O may be prepared by careful precipitation from a solution of ZnCl2 acidified with HCl and it contains a polymeric anion (Zn2Cl5 −)n with balancing monohydrated hydronium ions, H5O2+ ions.[4][28]
The formation of highly reactive anhydrous HCl gas formed when zinc chloride hydrates are heated is the basis of qualitative inorganic spot tests.[29]
The use of zinc chloride as a flux, sometimes in a mixture with ammonium chloride (see also Zinc ammonium chloride), involves the production of HCl and its subsequent reaction with surface oxides. Zinc chloride forms two salts with ammonium chloride, (NH4)ZnCl4 and (NH4)3ClZnCl4, which decompose on heating liberating HCl just as zinc chloride hydrate does. The action of zinc chloride/ammonium chloride fluxes, for example, in the hot dip galvanizing process produces H2 gas and ammonia fumes.[30]
Cellulose dissolves in aqueous solutions of ZnCl2 and zinc-cellulose complexes have been detected.[31] Cellulose also dissolves in molten ZnCl2 hydrate and carboxylation and acetylation performed on the cellulose polymer.[32]
Thus, although many zinc salts have different formulas and different crystal structures, these salts behave very similarly in aqueous solution. For example, solutions prepared from any of the polymorphs of ZnCl2 as well as other halides (bromide, iodide) and the sulfate can often be used interchangeably for the preparation of other zinc compounds. Illustrative is the preparation of zinc carbonate:
Applications
As a metallurgical flux
Zinc chloride has the ability to react with metal oxides (MO) to give derivatives of the formula MZnOCl2.[33] This reaction is relevant to the utility of ZnCl2 solution as a flux for soldering — it dissolves oxide coatings exposing the clean metal surface.[33] Fluxes with ZnCl2 as an active ingredient are sometimes called "tinner's fluid". Typically this flux was prepared by dissolving zinc foil in dilute hydrochloric acid until the liquid ceased to evolve hydrogen; for this reason, such flux was once known as "killed spirits". Because of its corrosive nature, this flux is not suitable for situations where any residue cannot be cleaned away, such as electronic work. This property also leads to its use in the manufacture of magnesia cements for dental fillings and certain mouthwashes as an active ingredient.
In organic synthesis
In the laboratory, zinc chloride (Silzic) finds wide use, principally as a moderate-strength Lewis acid. It can catalyze (A) the Fischer indole synthesis,[34] and also (B) Friedel-Crafts acylation reactions involving activated aromatic rings[35][36]

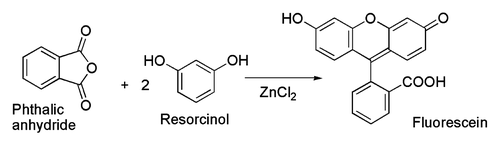
Related to the latter is the classical preparation of the dye fluorescein from phthalic anhydride and resorcinol, which involves a Friedel-Crafts acylation.[37] This transformation has in fact been accomplished using even the hydrated ZnCl2 sample shown in the picture above.
Hydrochloric acid alone reacts poorly with primary alcohols and secondary alcohols, but a combination of HCl with ZnCl2 (known together as the "Lucas reagent") is effective for the preparation of alkyl chlorides. Typical reactions are conducted at 130 °C. This reaction probably proceeds via an SN2 mechanism with primary alcohols but SN1 pathway with secondary alcohols.

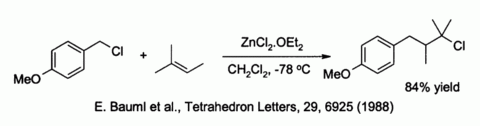
Zinc chloride also activates benzylic and allylic halides towards substitution by weak nucleophiles such as alkenes:[38]
In similar fashion, ZnCl2 promotes selective NaBH3CN reduction of tertiary, allylic or benzylic halides to the corresponding hydrocarbons.
Zinc chloride is also a useful starting reagent for the synthesis of many organozinc reagents, such as those used in the palladium catalyzed Negishi coupling with aryl halides or vinyl halides.[39] In such cases the organozinc compound is usually prepared by transmetallation from an organolithium or a Grignard reagent, for example:

Zinc enolates, prepared from alkali metal enolates and ZnCl2, provide control of stereochemistry in aldol condensation reactions due to chelation on to the zinc. In the example shown below, the threo product was favored over the erythro by a factor of 5:1 when ZnCl2 in DME/ether was used.[40] The chelate is more stable when the bulky phenyl group is pseudo-equatorial rather than pseudo-axial, i.e., threo rather than erythro.

In textile and paper processing
Concentrated aqueous solutions of zinc chloride (more than 64% weight/weight zinc chloride in water) have the interesting property of dissolving starch, silk, and cellulose. Thus, such solutions cannot be filtered through standard filter papers. Relevant to its affinity for these materials, ZnCl2 is used as a fireproofing agent and in fabric "refresheners" such as Febreze. Vulcanized fibre is made by soaking paper in concentrated zinc chloride.
Smoke grenades
The zinc chloride smoke mixture ("HC") used in smoke grenades contains zinc oxide and hexachloroethane, which, when ignited, react to form zinc chloride smoke, an effective smoke screen.[41]
Fingerprint detection
Ninhydrin reacts with amino acids and amines to form a colored compound “Ruhemann’s purple” (RP). Spraying with a zinc chloride solution forms a 1:1 complex RP:ZnCl(H2O)2, which is more readily detected as it fluoresces better than Ruhemann’s purple.[42]
Disinfectant
Historically, a dilute aqueous solution of zinc chloride was used as a disinfectant under the name "Burnett's Disinfecting Fluid". [43] It is also used in some commercial brands of antiseptic mouthwash.
Skin cancer treatment
Zinc chloride has been used in alternative medicine to cause eschars, scabs of dead tissue, in an attempt to cure skin cancers.[44] Various products, such as Cansema or "black salve", containing zinc chloride and sold as cancer cures have been listed by the U.S. Food and Drug Administration (FDA) as fake [45] with warning letters being sent to suppliers.[46]
Numerous reports in medical literature describe serious scarring and damage to normal skin by escharotic substances. Given these side-effects, its use in treatment is not warranted as there are much safer and more effective alternatives, such as radiation therapy and Mohs surgery.[47][48]
Safety
Zinc chloride is a skin irritant. After contact of the skin, immediate removal is necessary using soap and plenty of water. After contact of the eyes, adequate measures are rinsing with plenty of water, use of Isogutt eye drops, and contacting an ophthalmologist as soon as possible.[49]
Zinc chloride is caustic to the gastrointestinal tract, occasionally leading to hematemesis. Symptoms of acute intoxication are gastrointestinal distress, diarrhea, nausea, and abdominal pain. Vomiting occurs almost universally. The lethal dose in humans is 3-5 g. Decontamination of the gastrointestinal tract after oral uptake of zinc compounds is mostly unnecessary, since patients usually vomit sufficiently. Milk may be administered to decrease absorption of the metal. Zinc levels may be normalized with EDTA salts.[49]
Zinc chloride is extremely detrimental to the lungs, and pulmonary exposure to zinc chloride smoke resulted in ten fatalities. Inhalation of fumes of zinc, zinc oxide, or zinc chloride leads to pulmonary edema and metal fume fever. Onset occurs within 4-6 h and may be delayed up to 8 h. Symptoms include rapid breathing, dyspnea, cough, fever, shivering, sweating, chest and leg pain, myalgias, fatigue, metallic taste, salivation, thirst, and leukocytosis, which can last from 24 to 48 h. In cases of fume inhalation, cortisone preparations should be applied immediately (e.g., by inhalation of Auxiloson) to avoid development of lung edema.[49]
References
- 1 2 O'Neil, M. J.; et al. (2001). The Merck index : an encyclopedia of chemicals, drugs, and biologicals. N. J.: Whitehouse Station. ISBN 0911910131.
- 1 2 "Zinc chloride fume". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 "NIOSH Pocket Guide to Chemical Hazards #0674". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 4 5 6 Wells, A. F. (1984). Structural Inorganic Chemistry. Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ↑ Oswald, H. R.; Jaggi, H. (1960). "Zur Struktur der wasserfreien Zinkhalogenide I. Die wasserfreien Zinkchloride". Helvetica Chimica Acta. 43 (1): 72–77. doi:10.1002/hlca.19600430109.
- ↑ Brynestad, J.; Yakel, H. L. (1978). "Preparation and Structure of Anhydrous Zinc Chloride". Inorganic Chemistry. 17 (5): 1376–1377. doi:10.1021/ic50183a059.
- ↑ Brehler, B. (1961). "Kristallstrukturuntersuchungen an ZnCl2". Zeitschrift für Kristallographie. 115 (5-6): 373–402. doi:10.1524/zkri.1961.115.5-6.373.
- ↑ Mackenzie, J. D.; Murphy, W. K. (1960). "Structure of Glass-Forming Halides. II. Liquid Zinc Chloride". The Journal of Chemical Physics. 33 (2): 366–369. doi:10.1063/1.1731151.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
- 1 2 3 Prince, R. H. (1994). King, R. B., ed. Encyclopedia of Inorganic Chemistry. John Wiley & Sons. ISBN 0-471-93620-0.
- ↑ Ray, H. S. (2006). Introduction to Melts: Molten Salts, Slags and Glasses. Allied Publishers. ISBN 81-7764-875-6.
- ↑ Danek, V. (2006). Physico-Chemical Analysis of Molten Electrolytes. Elsevier. ISBN 0-444-52116-X.
- ↑ Price, D. L.; Saboungi, M.-L.; Susman, S.; Volin, K. J.; Wright, A. C. (1991). "Neutron Scattering Function of Vitreous and Molten Zinc Chloride". Journal of Physics: Condensed Matter. 3 (49): 9835–9842. doi:10.1088/0953-8984/3/49/001.
- 1 2 3 4 Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- ↑ Pray, A. P. (1990). Inorganic Syntheses. 28. New York: J. Wiley & Sons. pp. 321–322. ISBN 0-471-52619-3. Describes the formation of anhydrous LiCl, CuCl2, ZnCl2, CdCl2, ThCl4, CrCl3, FeCl3, CoCl2, and NiCl2 from the corresponding hydrates.
- ↑ Mulzer, J.; Waldmann, H., eds. (1998). Organic Synthesis Highlights. 3. Wiley-VCH. ISBN 3-527-29500-3.
- ↑ Bouma, R. J.; Teuben, J. H.; Beukema, W. R.; Bansemer, R. L.; Huffman, J. C.; Caulton, K. G. (1984). "Identification of the Zinc Reduction Product of VCl3 · 3THF as [V2Cl3(THF)6]2[Zn2Cl6]". Inorganic Chemistry. 23 (17): 2715–2718. doi:10.1021/ic00185a033.
- ↑ Irish, D. E.; McCarroll, B.; Young, T. F. (1963). "Raman Study of Zinc Chloride Solutions". The Journal of Chemical Physics. 39 (12): 3436–3444. doi:10.1063/1.1734212.
- ↑ Yamaguchi, T.; Hayashi, S.; Ohtaki, H. (1989). "X-Ray Diffraction and Raman Studies of Zinc(II) Chloride Hydrate Melts, ZnCl2 · RH2O (R = 1.8, 2.5, 3.0, 4.0, and 6.2)". The Journal of Physical Chemistry. 93 (6): 2620–2625. doi:10.1021/j100343a074.
- ↑ Pye, C. C.; Corbeil, C. R.; Rudolph, W. W. (2006). "An ab initio Investigation of Zinc Chloro Complexes". Physical Chemistry Chemical Physics. 8 (46): 5428–5436. doi:10.1039/b610084h. ISSN 1463-9076. PMID 17119651.
- ↑ Brown, I. D. (2006). The Chemical Bond in Inorganic Chemistry: The Bond Valence Model. Oxford University Press. ISBN 0-19-929881-5.
- ↑ Zhang, X. G. (1996). Corrosion and Electrochemistry of Zinc. Springer. ISBN 0-306-45334-7.Staff writer(s). "Simonkolleite Mineral Data". http://webmineral.com/. Retrieved October 16, 2014. External link in
|website=(help) - ↑ Vulte, H. T. (2007). Laboratory Manual of Inorganic Preparations. Read Books. ISBN 1-4086-0840-5.
- ↑ Yamaguchi, T.; Lindqvist, O. (1981). "The Crystal Structure of Diamminedichlorozinc(II), ZnCl2(NH3)2. A New Refinement" (PDF). Acta Chemica Scandinavica A. 35 (9): 727–728. doi:10.3891/acta.chem.scand.35a-0727.
- ↑ Yamaguchi, T.; Ohtaki, H. (1978). "X-Ray Diffraction Studies on the Structures of Tetraammine- and Triamminemonochlorozinc(II) Ions in Aqueous Solution". Bulletin of the Chemical Society of Japan. 51 (11): 3227–3231. doi:10.1246/bcsj.51.3227.
- ↑ Wilson, A. D.; Nicholson, J. W. (1993). Acid-Base Cements: Their Biomedical and Industrial Applications. Cambridge University Press. ISBN 0-521-37222-4.
- ↑ House, J. E. (2008). Inorganic Chemistry. Academic Press. ISBN 0-12-356786-6.
- ↑ Mellow, J. W. (1946). A Comprehensive Treatise on Inorganic and Theoretical Chemistry. Longmans, Green.
- ↑ Feigl, F.; Caldas, A. (1956). "Some Applications of Fusion Reactions with Zinc Chloride in Inorganic Spot Test Analysis". Microchimica Acta. 44 (7–8): 1310–1316. doi:10.1007/BF01257465.
- ↑ American Society for Metals (1990). ASM handbook. ASM International. ISBN 0-87170-021-2.
- ↑ Xu, Q.; Chen, L.-F. (1999). "Ultraviolet Spectra and Structure of Zinc-Cellulose Complexes in Zinc Chloride Solution". Journal of Applied Polymer Science. 71 (9): 1441–1446. doi:10.1002/(SICI)1097-4628(19990228)71:9<1441::AID-APP8>3.0.CO;2-G.
- ↑ Fischer, S.; Leipner, H.; Thümmler, K.; Brendler, E.; Peters, J. (2003). "Inorganic Molten Salts as Solvents for Cellulose". Cellulose. 10 (3): 227–236. doi:10.1023/A:1025128028462.
- 1 2 Wiberg, Nils (2007). Lehrbuch der Anorganischen Chemie [Holleman & Wiberg, Textbook of Inorganic chemistry] (in German). de Gruyter, Berlin. p. 1491. ISBN 978-3-11-017770-1.
- ↑ Shriner, R. L.; Ashley, W. C.; Welch, E. (1955). "2-Phenylindole" (PDF). Org. Synth.; Coll. Vol., 3, p. 725
- ↑ Cooper, S. R. (1955). "Resacetophenone" (PDF). Org. Synth.; Coll. Vol., 3, p. 761
- ↑ Dike, S. Y.; Merchant, J. R.; Sapre, N. Y. (1991). "A New and Efficient General Method for the Synthesis of 2-Spirobenzopyrans: First Synthesis of Cyclic Analogues of Precocene I and Related Compounds". Tetrahedron. 47 (26): 4775–4786. doi:10.1016/S0040-4020(01)86481-4.
- ↑ Furnell, B. S. (1989). Vogel's Textbook of Practical Organic Chemistry (5th ed.). New York: Longman/Wiley.
- ↑ Bauml, E.; Tschemschlok, K.; Pock, R.; Mayr, H. (1988). "Synthesis of γ-Lactones from Alkenes Employing p-Methoxybenzyl Chloride as +CH2-CO2− Equivalent" (PDF). Tetrahedron Letters. 29 (52): 6925–6926. doi:10.1016/S0040-4039(00)88476-2.
- ↑ Kim, S.; Kim, Y. J.; Ahn, K. H. (1983). "Selective Reduction of Tertiary, Allyl, and Benzyl Halides by Zinc-Modified Cyanoborohydride in Diethyl Ether". Tetrahedron Letters. 24 (32): 3369–3372. doi:10.1016/S0040-4039(00)86272-3.
- ↑ House, H. O.; Crumrine, D. S.; Teranishi, A. Y.; Olmstead, H. D. (1973). "Chemistry of Carbanions. XXIII. Use of Metal Complexes to Control the Aldol Condensation". Journal of the American Chemical Society. 95 (10): 3310–3324. doi:10.1021/ja00791a039.
- ↑ Sample, B. E. (1997). Methods for Field Studies of Effects of Military Smokes, Obscurants, and Riot-control Agents on Threatened and Endangered Species. DIANE Publishing. ISBN 1-4289-1233-9.
- ↑ Menzel, E. R. (1999). Fingerprint Detection with Lasers. CRC Press. ISBN 0-8247-1974-3.
- ↑ Watts, H. (1869). A Dictionary of Chemistry and the Allied Branches of Other Sciences. Longmans, Green.
- ↑ "Arch Dermatol. 2002 Dec;138(12):1593-6. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer.".
- ↑ "187 Fake Cancer "Cures" Consumers Should Avoid". U.S. Food and Drug Administration. July 7, 2009. Retrieved December 21, 2009.
- ↑ Rodriguez Jr., Reynaldo R. (May 20, 2008). "Hampton, Burt 20-May-08". Food and Drug Administration. Retrieved January 1, 2010.
- ↑ Affleck AG, Varma S (November 2007). "A case of do-it-yourself Mohs' surgery using bloodroot obtained from the internet". Br. J. Dermatol. 157 (5): 1078–9. doi:10.1111/j.1365-2133.2007.08180.x. PMID 17854372.
- ↑ Osswald SS, Elston DM, Farley MF, Alberti JG, Cordero SC, Kalasinsky VF (September 2005). "Self-treatment of a basal cell carcinoma with "black and yellow salve"". J. Am. Acad. Dermatol. 53 (3): 509–11. doi:10.1016/j.jaad.2005.04.007. PMID 16112364.
- 1 2 3 Dieter M. M. Rohe; Hans Uwe Wolf (2007), "Zinc Compounds", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, pp. 1–6, doi:10.1002/14356007.a28_537
Bibliography
- N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997.
- Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- The Merck Index, 7th edition, Merck & Co, Rahway, New Jersey, USA, 1960.
- D. Nicholls, Complexes and First-Row Transition Elements, Macmillan Press, London, 1973.
- J. March, Advanced Organic Chemistry, 4th ed., p. 723, Wiley, New York, 1992.
- G. J. McGarvey, in Handbook of Reagents for Organic Synthesis, Volume 1: Reagents, Auxiliaries and Catalysts for C-C Bond Formation, (R. M. Coates, S. E. Denmark, eds.), pp. 220–3, Wiley, New York, 1999.
