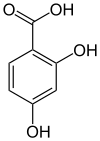
2,4-Dihydroxybenzoic acid
 | |
| Names | |
|---|---|
| IUPAC name
2,4-dihydroxybenzoic acid | |
| Other names
beta-Resorcylic acid beta-Resorcinolic acid p-Hydroxysalicylic acid 2,4-DHBA | |
| Identifiers | |
| 89-86-1 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL328910 |
| ChemSpider | 1446 |
| DrugBank | DB02839 |
| ECHA InfoCard | 100.001.770 |
| PubChem | 1491 |
| |
| |
| Properties | |
| C7H6O4 | |
| Molar mass | 154.12 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
2,4-Dihydroxybenzoic acid (β-resorcylic acid) is a dihydroxybenzoic acid.
As a resorcylic acid, it is one of the three isomeric crystalline acids that are both carboxyl derivatives of resorcinol and dihydroxy derivatives of benzoic acid.[1]
It is a degradation product of cyanidin glycosides from tart cherries in cell cultures.[2] It is also a metabolite found in human plasma after cranberry juice consumption.[3]
References
- ↑ Resorcyclic acid on merriam-webster on-line dictionary
- ↑ Degradation Products of Cyanidin Glycosides from Tart Cherries and Their Bioactivities. Navindra P. Seeram, Leslie D. Bourquin and Muraleedharan G. Nair, J. Agric. Food Chem., 2001, 49 (10), pp. 4924–4929, doi:10.1021/jf0107508
- ↑ GC-MS Determination of Flavonoids and Phenolic and Benzoic Acids in Human Plasma after Consumption of Cranberry Juice. Kai Zhang and Yuegang Zuo, J. Agric. Food Chem., 2004, 52 (2), pp. 222–227, doi:10.1021/jf035073r
This article is issued from Wikipedia - version of the 7/7/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.