Non-Kekulé molecule

A non-Kekulé molecule is a conjugated hydrocarbon that cannot be assigned a classical Kekulé structure.
Since non-Kekulé molecules have two or more formal radical centers, their spin-spin interactions can cause electrical conductivity or ferromagnetism (molecule-based magnets), and applications to functional materials are expected. However, as these molecules are quite reactive and most of them are easily decomposed or polymerized at room temperature, strategies for stabilization are needed for their practical use. Synthesis and observation of these reactive molecules are generally accomplished by matrix-isolation methods.
Biradicals
The simplest non-Kekulé molecules are biradicals. A biradical is an even-electron chemical compound with two free radical centres which act independently of each other. They should not be confused with the more general class of diradicals.[1]
One of the first biradicals was synthesized by Wilhelm Schlenk in 1915 following the same methodology as Moses Gomberg's triphenylmethyl radical. The so-called Schlenk-Brauns hydrocarbons are:[2]

Eugene Müller, with the aid of a Gouy balance, established for the first time that these compounds are paramagnetic with a triplet ground state.
Another classic biradical was synthesised by Tschitschibabin in 1907.[3][4] Other classical examples are the biradicals described by Yang in 1960[5] and by Coppinger in 1962.[6][7][8]
 |
 |  | ||
| Tschitschibabin biradical (1907) | Yang biradical (1960) | Coppinger biradical 1962 | ||
Trimethylenemethane
A well studied biradical is trimethylenemethane (TMM), C
4H
6. In 1966 Paul Dowd determined with electron spin resonance that this compound also has a triplet state. In a crystalline host the 6 hydrogen atoms in TMM are identical.
Quinodimethanes and PAHs
Other examples of non-Kekulé molecules are the biradicaloid quinodimethanes, that have a six-membered ring with methylene substituents.
Non-Kekulé polynuclear aromatic hydrocarbons are composed of several fused six-membered rings. The simplest member of this class is triangulene. After unsuccessful attempts by Erich Clar in 1953, trioxytriangulene was synthesized by Richard J. Bushby in 1995, and kinetically stabilized triangulene by Kazuhiro Nakasuji in 2001. A related class of biradicals are para-benzynes.
Other studied biradicals are those based on pleiadene,[9] extended viologens,[10][11] corannulenes,[12] nitronyl-nitroxide,[13] bis(phenalenyl)s [14] and teranthenes.[15][16]
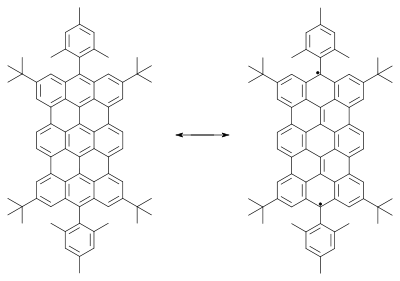 |
 | |
| Teranthene biradical Singlet. max. 3 stabilizing Clar sextets, stable rt, air. 50% biradical, molecular section of graphene | Bisphenalenyl biradical Singlet. max. 6 stabilizing Clar sextets, stable rt, air. 42% biradical | |
Pleiadene has been synthesised from acenaphthylene and anthranilic acid / amyl nitrite:
 |
| Pleiadene generation and dimerization |
Oxyallyl
The oxyallyl diradical (OXA) is a trimethylenemethane molecule with one methylene group replaced by oxygen. This reactive intermediate is postulated to occur in ring opening of cyclopropanones, allene oxides and in the Favorskii rearrangement. The intermediate has been produced by reaction of oxygen radical anions with acetone and studied by photoelectron spectroscopy.[17] The experimental electron affinity of OXA is 1.94 eV.
Classification

Non-Kekulé molecules with two formal radical centers (non-Kekulé diradicals) can be classified into non-disjoint and disjoint by the shape of their two non-bonding molecular orbitals (NBMOs).
Both NBMOs of molecules with non-disjoint characteristics such as trimethylenemethane have electron density at the same atom. According to Hund's rule, each orbital is filled with one electron with parallel spin, avoiding the Coulomb repulsion by filling one orbital with two electrons. Therefore, such molecules with non-disjoint NBMOs are expected to prefer a triplet ground state.
In contrast, the NBMOs of the molecules with disjoint characteristics such as tetramethyleneethane can be described without having electron density at the same atom. With such MOs, the destabilization factor by the Coulomb repulsion becomes much smaller than with non-disjoint type molecules, and therefore the relative stability of the singlet ground state to the triplet ground state will be nearly equal, or even reversed because of exchange interaction.
References
- ↑ IUPAC Gold Book definitions of biradical and diradicals
- ↑ Robert A. Moss ed. (2004), "Reactive Intermediate Chemistry" (Book) Wiley-Interscience. ISBN 0-471-23324-2
- ↑ A. E. Tschitschibabin (1907), "Über einige phenylierte Derivate des p, p-Ditolyls." Berichte der Deutschen Chemischen Gesellschaft, volume 40, pages 1810–1819. doi:10.1002/cber.19070400282
- ↑ Lawrence K. Montgomery, John C. Huffman, Edward A. Jurczak, Martin P. Grendze Jr. (1986), "The molecular structures of Thiele's and Chichibabin's hydrocarbons." Journal of the American Chemical Society, volume 108, issue 19, pages 6004–6011 doi:10.1021/ja00279a056
- ↑ N. C. Yang and A. J. Castro (1960), "Synthesis of a stable biradical" n P. Grendze Jr. (1986), "The molecular structures of Thiele's and Chichibabin's hydrocarbons." Journal of the American Chemical Society, volume 82, issue 23, page 6208 doi:10.1021/ja01508a067
- ↑ G. M. Coppinger (1962), "A stable phenoxy radical inert to oxygen" Tetrahedron, volume 18, issue 1, pages 61-65. doi:10.1016/0040-4020(62)80024-6
- ↑ G. M. Coppinger (1964), "Inhibition Reactions of Hindered Phenols" Journal of the American Chemical Society, volume 86, issue 20, pages 4385–4388 doi:10.1021/ja01074a032
- ↑ M. Baumgarten (2003/2004), "High spin molecules directed towards molecular magnets", chapter 12 in "EPR of free radicals in solids, Trends in methods and application", A. Lund, M. Shiotani (eds), Kluwer, pages 491-528
- ↑ Jaroslav Kolc and Josef Michl (1973), "π,π-Biradicaloid hydrocarbons. Pleiadene family. I. Photochemical preparation from cyclobutene precursors." Journal of the American Chemical Society, volume 95, issue 22, pages 7391–7401 doi:10.1021/ja00803a030
- ↑ William W. Porter III, Thomas P. Vaid, and Arnold L. Rheingold (2005), "Synthesis and characterization of a highly reducing neutral “extended viologen” and the isostructural hydrocarbon 4,4''''-di-n-octyl-p-quaterphenyl" Journal of the American Chemical Society, volume 127, issue 47, pages 16559–16566 doi:10.1021/ja053084q
- ↑ J. Casado, S. Patchkovskii, M. Zgierski, L. Hermosilla, C. Sieiro, M. Moreno Oliva, and J. López Navarrete (2008), "Raman Detection of “ambiguous” conjugated biradicals: Rapid thermal singlet-to-triplet intersystem crossing in an extended viologen." Angewandte Chemie International Edition, volume 47, pages 1443–1446. doi:10.1002/anie.200704398
- ↑ A. Ueda, S. Nishida, K. Fukui, T. Ise, D. Shiomi, K. Sato, T. Takui, K. Nakasuji, and Y. Morita (2010), "Three-dimensional intramolecular exchange interaction in a curved and nonalternant π-conjugated system: Corannulene with two phenoxyl radicals." Angewandte Chemie International Edition, volume 49, pages 1678–1682. doi:10.1002/anie.200906666 PMID 20108294
- ↑ Strong Exchange Interactions between Two Radicals Attached to Nonaromatic Spacers Deduced from Magnetic, EPR, NMR, and Electron Density Measurements Raymond Ziessel Christophe Stroh, Henrike Heise, Frank H. Köhler, Philippe Turek,Nicolas Claiser, Mohamed Souhassou, and Claude Lecomte J. Am. Chem. Soc., 2004, 126 (39), pp 12604–12613 doi:10.1021/ja0305959
- ↑ Takashi Kubo, Akihiro Shimizu, Mikio Uruichi, Kyuya Yakushi, Masayoshi Nakano, Daisuke Shiomi, Kazunobu Sato, Takeji Takui, Yasushi Morita, Kazuhiro Nakasuji (2007), "Singlet biradical character of phenalenyl-based kekulé hydrocarbon with naphthoquinoid structure" Org. Lett., volume 9, issue 1, pages 81–84 doi:10.1021/ol062604z
- ↑ Akihito Konishi, Yasukazu Hirao, Masayoshi Nakano, Akihiro Shimizu, Edith Botek, Benot Champagne, Daisuke Shiomi, Kazunobu Sato, Takeji Takui, Kouzou Matsumoto, Hiroyuki Kurata and Takashi Kubo (2010), "Synthesis and characterization of teranthene: A singlet biradical polycyclic aromatic hydrocarbon having Kekulé structures." Journal of the American Chemical Society, volume 132, issue 32, pages 11021–11023 doi:10.1021/ja1049737
- ↑ Lambert, C. (2011), "Towards polycyclic aromatic hydrocarbons with a singlet open-shell ground state." Angewandte Chemie International Edition, volume 50, pages 1756–1758. doi:10.1002/anie.201006705
- ↑ Takatoshi Ichino, Stephanie M. Villano, Adam J. Gianola, Daniel J. Goebbert, Luis Velarde, Andrei Sanov, Stephen J. Blanksby, Xin Zhou, David A. Hrovat, Weston Thatcher Borden, and W. Carl Lineberger (2009), "The lowest singlet and triplet states of the oxyallyl diradical" Angewandte Chemie International Edition volume 48, pages 8509–8511 doi:10.1002/anie.200904417