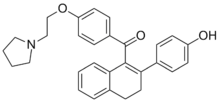
Trioxifene
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | 63619-84-1 |
| PubChem (CID) | 50139 |
| ChemSpider | 45471 |
| UNII |
R0130F043H |
| Chemical and physical data | |
| Formula | C30H31NO3 |
| Molar mass | 453.57 g/mol |
Trioxifene (LY-133,314) was a selective estrogen receptor modulator (SERM) with competitive binding activity against estradiol for estrogen receptor-alpha (ERα) and antagonistic activity against ERα-mediated gene expression, that was under preclinical and clinical development by Eli Lilly and Company for breast cancer and prostate cancer,[1] but was abandoned.[2]:11
References
- ↑ Neubauer BL, McNulty AM, Chedid M, Chen K, Goode RL, Johnson MA, Jones CD, Krishnan V, Lynch R, Osborne HE, Graff JR (September 2003). "The selective estrogen receptor modulator trioxifene (LY133314) inhibits metastasis and extends survival in the PAIII rat prostatic carcinoma model". Cancer Research. 63 (18): 6056–62. PMID 14522935.
- ↑ Philipp Y. Maximov, Russell E. McDaniel, V. Craig Jordan. Tamoxifen: Pioneering Medicine in Breast Cancer. Milestones in Drug Therapy. Springer Science & Business Media, 2013. ISBN 9783034806640
This article is issued from Wikipedia - version of the 6/6/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.