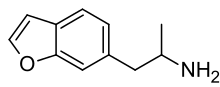
6-APB
 | |
 | |
| Clinical data | |
|---|---|
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
286834-85-3 |
| PubChem (CID) | 9794343 |
| ChemSpider |
7970110 |
| UNII |
285VE60914 |
| Chemical and physical data | |
| Formula | C11H13NO |
| Molar mass | 175.23 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
6-APB (6-(2-aminopropyl)benzofuran; see infobox for the correct IUPAC name) is an empathogenic psychoactive compound of the substituted benzofuran, substituted amphetamine and substituted phenethylamine classes. 6-APB and other compounds are sometimes informally called "Benzofury". It is similar in structure to MDA, but differs in that the 3,4-methylenedioxyphenyl ring system has been replaced with a benzofuran ring. 6-APB is also the unsaturated benzofuran derivative of 6-APDB. It may appear as a tan grainy powder. 6-APB was first synthesized by David Nichols and his team in 1993 while studying non-neurotoxic analogs of MDMA.
While the drug never became particularly popular, it briefly entered the rave and underground clubbing scene in the UK before its sale and import were banned. It falls under the category of research chemicals, sometimes called "legal highs." Because 6-APB and other substituted benzofurans have not been explicitly outlawed in some countries, they are often technically legal, contributing to their popularity.
Pharmacology
Pharmacodynamics
6-APB is a triple monoamine reuptake inhibitor with Ki values of 117, 150 and 2698 nM for NET, DAT and SERT respectively as well as being a potent agonist for the 5-HT2B receptor (Ki 3.7 nM).[1] The subjective effects and structure–activity relationship suggest that it is also a releasing agent.
The agonism for 5-HT2B makes it likely that 6-APB would be cardiotoxic with long term use, as seen in other 5-HT2B agonists, such as fenfluramine and MDMA.[1][2] 6-APB acts as an agonist of the 5-HT2C receptor,[3] which may be responsible for its appetite suppression. While 6-APB is an agonist at all three 5-HT2 receptor subtypes, its affinity for 5-HT2B is significantly higher. It is both more potent and more selective over other serotonin receptors than the reference agonist, BW723C86, which is commonly used for research into 5-HT2B receptors.
Pharmacokinetics
The pharmacokinetics of 6-APB have not been studied, however, some information can be extracted from user reports. These suggest an slow onset of 20–120 minutes. The drugs maximum effects last 3–4 hours, followed by a comedown phase of up to 24 hours.[4]
Metabolism
Although limited literature is available, there is some data on metabolism of 6-APB in rats. Its Phase I metabolism involves hydroxylation of the furan ring, then cleavage of the ring, followed by a reduction of the unsaturated aldehyde from the previous step. The resulting aldehyde may then takes two paths. It is either oxidized to a carboxylic acid or reduced to an alcohol, and then hydroxylated. Phase II metabolism consists of glucuronidation. The most prevalent metabolites in rats were 3-carboxymethyl-4-hydroxyamphetamine and 4-carboxymethyl-3-hydroxyamphetamine.[5]
Chemistry
6-APB and its structural isomer 5-APB have been tested with a series of agents including: Marquis, Liebermann, Mecke, and Froehde reagents. Exposing compounds to the reagents gives a colour change which is indicative of the compound under test.
| Compound | Marquis | Liebermann | Mecke | Froehde |
|---|---|---|---|---|
| 5-APB | Black | Black | Black | Dark Purple |
| 6-APB | Purple | Dark Purple | Purple | Purple |
6-APB succinate is reported to be practically insoluble in CHCl3 as well as very minimally soluble in cold water. A batch seized by the DEA contained a 2:1 ratio of succinate to 6-APB.[6]
Synthesis
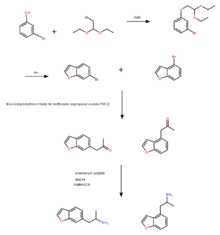
Exact experimental details are not provided, but roughly follow the provided procedure. The procedure of Briner et al.[3] was the most comprehensive thus far and was replicated by Casale and Hays[6] as described below.
Briefly, 3-bromophenol was refluxed with bromoacetaldehyde and NaH to give the diethyl acetyl, which then was heated with polyphosphoric acid to give a mixture of bromobenzofurans structural isomers: 4-Bromo-1-benzofuran and 6-Bromo-1-benzofuran. Both isomers were separated via silica gel column chromatography, then catalytically converted to their respective 2-propanones, and then reductively aminated to 6-APB and 4-APB. Both of which were converted to their HCl ion-pairs for further examination.
Effects
Users report a feeling a sense of euphoria and energy. The effect has been most closely compared with those of ecstasy.[7] Adverse effects include loss of appetite, hallucinations, severe paranoia, tachycardia, insomnia, and jaw tensions. Users warn of neurotoxicity and brain damage, although the reports are unconfirmed. Web forum users also report serotonin syndrome, especially if combined with SSRI's or MAOI's. These side effects are similar to MDA, but much more profound than MDMA. Researchers claim that it produces about the same addiction as amphetamines.[7]
Law
Canada
6-APB is Schedule III[8] in Canada as it is an analogue of MDA.[9] The CDSA was updated as a result of the Safe Streets Act changing amphetamines from Schedule 3 to Schedule 1.[10]
France
6-APB is unscheduled in France.
Italy
6-APB is illegal in Italy.[11]
Germany
6-APB is illegal in Germany since the 17th of July, 2013, when it was added to Anlage II of the Betäubungsmittelgesetz.
Netherlands
6-APB is not listed under the Opium Law or the Medicine Act in the Netherlands, and thus currently legal.
New Zealand and Australia
Certain countries contain a "substantially similar" catch-all clause in their drug law, such as New Zealand and Australia. This includes 6-APB as it is similar in chemical structure to the class A drug MDA, meaning 6-APB may be viewed as a controlled substance analogue in these jurisdictions.[12]
Sweden
In Sweden, as of 27 December 2009 6-APB is classified as "health hazard" under the act Lagen om förbud mot vissa hälsofarliga varor (translated Act on the Prohibition of Certain Goods Dangerous to Health).[13]
UK
On June 10, 2013 6-APB and a number of analogues were classified as Temporary Class Drugs in the UK following an ACMD recommendation.[2] This means that sale and import of the named substances are criminal offences and are treated as for class B drugs.[14] On November 28, 2013 the ACMD recommended that 6-APB and related benzofurans should become Class B, Schedule 1 substances.[2] On March 5, 2014 the UK Home Office announced that 6-APB would be made a class B drug on 10 June 2014 alongside every other benzofuran entactogen and many structurally related drugs.[15]
USA
6-APB is unscheduled in the United States, but not currently approved by the Food and Drug Administration for human consumption.
References
- 1 2 Iversen, L.; Gibbons, S.; Treble, R.; Setola, V.; Huang, X. P.; Roth, B. L. (January 2013). "Neurochemical profiles of some novel psychoactive substances". European Journal of Pharmacology. 700 (1–3). doi:10.1016/j.ejphar.2012.12.006. PMC 3582025
 . PMID 23261499.
. PMID 23261499. - 1 2 3 Advisory Council on the Misuse of Drugs, Jeremy Browne (4 June 2013). "Temporary class drug order on benzofury and NBOMe compounds - letter from ACMD". GOV.UK.
- 1 2 US patent 7045545, Karin Briner, Joseph Paul Burkhart, Timothy Paul Burkholder, Matthew Joseph Fisher, William Harlan Gritton, Daniel Timothy Kohlman, Sidney Xi Liang, Shawn Christopher Miller, Jeffrey Thomas Mullaney, Yao-Chang Xu, Yanping Xu, "Aminoalkylbenzofurans as serotonin (5-HT(2c)) agonists", published 19 January 2000, issued 16 May 2006
- ↑ Shaun L. Greene (2013). Novel Psychoactive Substances: Classification, Pharmacology and Toxicology Chapter 16 – Benzofurans and Benzodifurans. Boston: Academic Press. pp. 383–392. doi:10.1016/B978-0-12-415816-0.00016-X. ISBN 9780124158160.
- ↑ Johanna J. Nugteren-van Lonkhuyzen; Antoinette J.H.P. van Riel; Tibor M. Brunt; Laura Hondebrink (December 2015). "Pharmacokinetics, pharmacodynamics and toxicology of new psychoactive substances (NPS): 2C-B, 4-fluoroamphetamine and benzofurans". Drug & Alcohol Dependence. 157: 18–27. doi:10.1016/j.drugalcdep.2015.10.011. PMID 26530501.
- 1 2 John F. Casale, Patrick A. Hays (January 2012). "The Characterization of 6-(2-Aminopropyl)benzofuran and Differentiation from its 4-, 5-, and 7-Positional Analogues" (PDF). Microgram Journal. 9 (2): 61–74.
- 1 2 "Legal Benzo Fury 'as dangerous as illegal drugs such as ecstasy', study claims". Dailymail.
- ↑ "Controlled Drugs and Substances Act: Legislative History · Schedule I". Isomer Design.
- ↑ "Controlled Drugs and Substances Act: Definitions and Interpretations". Isomer Design.
- ↑ "Safe Streets and Communities Act". Justice Laws Website. 2012.
- ↑ "Nuove tabelle delle sostanze stupefacenti e psicotrope (DPR 309/90)" (PDF) (in Italian). Ministero della Salute.
- ↑ "Misuse of Drugs Act 1975". New Zealand Legislation. 7 November 2015.
- ↑ "Förordning (1999:58) om förbud mot vissa hälsofarliga varor" (in Swedish). Lagbevakning med Notisum och Rättsnätet. 24 November 2009.
- ↑ Home Office, Jeremy Browne (4 June 2013). "'NBOMe' and 'Benzofury' banned". GOV.UK.
- ↑ UK Home Office (28 April 2014). "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014". The National Archives.