2-MDP
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | 33887-05-7 |
| PubChem (CID) | 26538 |
| ChemSpider |
24719 |
| Chemical and physical data | |
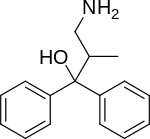
| Formula | C16H19NO |
| Molar mass | 241.33 g·mol−1 |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
2-MDP (U-23807A) is a dissociative anaesthetic drug which has been found to be an NMDA antagonist and produces similar effects to PCP in animals. The levo or (-) isomer is the active form of the drug.[1][2] It also has stimulant effects, having only around one third the potency of amphetamine by weight, but with a long duration of action, lasting more than 24 hours from a single oral dose.[3]
References
- ↑ Tang AH, Cangelosi AA, Code RA, Franklin SR. Phencyclidine-like behavioral effects of 2-methyl-3,3-diphenyl-3-propanolamine (2-MDP). Pharmacology, Biochemistry and Behaviour. 1984 Feb;20(2):209-13.
- ↑ Blake JC, Davies SN, Church J, Martin D, Lodge D. 2-Methyl-3,3-diphenyl-3-propanolamine (2-MDP) selectively antagonises N-methyl-aspartate (NMA). Pharmacology, Biochemistry and Behaviour. 1986 Jan;24(1):23-5.
- ↑ John H Biel. Annual Reports in Medicinal Chemistry, Volume 2. Academic Press 1966. p18.
| Receptor (ligands) |
| ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (blockers) |
| ||||||||||||||||||||||||||||||||||||||||||
| Enzyme (inhibitors) |
| ||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||
See also: GABAergics • GHBergics • Glycinergics | |||||||||||||||||||||||||||||||||||||||||||
Stimulants (category) | |
|---|---|
| Adamantanes |
|
| Adenosine antagonists | |
| Alkylamines | |
| Ampakines | |
| Arylcyclohexylamines | |
| Benzazepines | |
| Cholinergics |
|
| Convulsants | |
| Eugeroics | |
| Oxazolines | |
| Phenethylamines |
|
| Phenylmorpholines | |
| Piperazines | |
| Piperidines |
|
| Pyrrolidines | |
| Racetams | |
| Tropanes |
|
| Tryptamines | |
| Others |
|
This article is issued from Wikipedia - version of the 4/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.