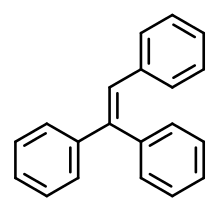
Triphenylethylene
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | 58-72-0 |
| PubChem (CID) | 6025 |
| ChemSpider | 5803 |
| Chemical and physical data | |
| Formula | C20H16 |
| Molar mass | 256.34104 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Triphenylethylene (TPE) is a simple aromatic hydrocarbon that possesses weak estrogenic activity.[1][2] Its estrogenic effects were discovered in 1937.[3]
TPE is the parent compound of a group of non-steroidal selective estrogen receptor modulators (SERMs) that includes tamoxifen, clomiphene, estrobin, broparestrol, toremifene, droloxifene, idoxifene, ospemifene, fispemifene, and miproxifene, among others.[1][2][4] The estrogen chlorotrianisene and the antiestrogen ethamoxytriphetol are also TPEs.
TPE and its SERM derivatives were derived from structural modification of diethylstilbestrol.[5]
See also
- Benzothiophene – parent compound for another group of non-steroidal SERMs that includes raloxifene
- Stilbestrol – parent compound of a group of non-steroidal estrogens that includes diethylstilbestrol
- Phenanthrene – parent compound of steroidal estrogens like estradiol
- Chrysene – parent compound of a group of non-steroidal weak estrogens that includes 2,8-DHHHC and tetrahydrochrysene
- Doisynolic acid – parent compound of a group of non-steroidal estrogens that includes doisynoestrol
- Allenolic acid – parent compound of a group of non-steroidal estrogens that includes methallenestril
References
- 1 2 JORDAN V. CRAIG; B.J.A. Furr (5 February 2010). Hormone Therapy in Breast and Prostate Cancer. Springer Science & Business Media. pp. 95–. ISBN 978-1-59259-152-7.
- 1 2 Philipp Y. Maximov; Russell E. McDaniel; V. Craig Jordan (23 July 2013). Tamoxifen: Pioneering Medicine in Breast Cancer. Springer Science & Business Media. pp. 4–. ISBN 978-3-0348-0664-0.
- ↑ Jie Jack Li (3 April 2009). Triumph of the Heart: The Story of Statins. Oxford University Press, USA. pp. 33–. ISBN 978-0-19-532357-3.
- ↑ Antonio Cano; Joacquim Calaf i Alsina; Jose Luis Duenas-Diez (22 September 2006). Selective Estrogen Receptor Modulators: A New Brand of Multitarget Drugs. Springer Science & Business Media. pp. 52–. ISBN 978-3-540-34742-2.
- ↑ Carmen Avendano; J. Carlos Menendez (11 June 2015). Medicinal Chemistry of Anticancer Drugs. Elsevier Science. pp. 87–. ISBN 978-0-444-62667-7.
This article is issued from Wikipedia - version of the 10/9/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.